Monomyristin and Monopalmitin Derivatives: Synthesis and Evaluation as Potential Antibacterial and Antifungal Agents
Abstract
:1. Introduction
2. Results and Discussion
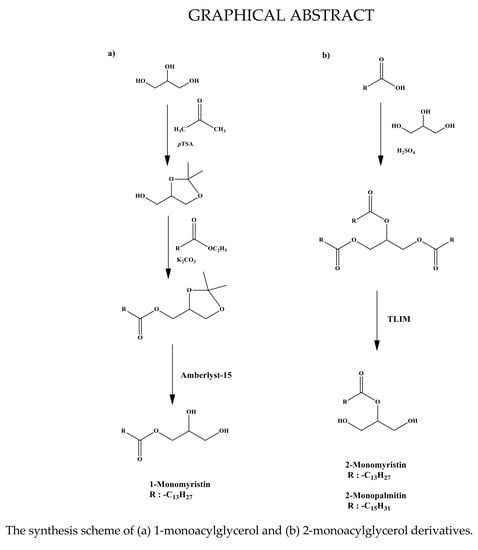
2.1. Synthesis of 1-Monomyristin
2.2. Synthesis of 2-Monomyristin and 2-Monopalmitin
2.3. Antibacterial and Antifungal Assays of Products
3. Materials and Methods
3.1. Materials
3.2. Equipment
3.3. Synthesis of 1-Monomyristin
3.3.1. Synthesis of 2,2-Dimethyl-1,3-dioxolan-4-methanol (1,2-O-isopropylidene glycerol)
3.3.2. Synthesis of Ethyl Myristate
3.3.3. Synthesis of Isopropylidene Glycerol Myristate
3.3.4. Synthesis of 1-Monomyristin
3.4. Synthesis of 2-Monomyristin
3.4.1. Synthesis of Trimyristin
3.4.2. Synthesis of 2-Monomyristin
3.5. Synthesis of 2-Monopalmitin
3.5.1. Synthesis of Tripalmitin
3.5.2. Synthesis of 2-Monopalmitin
3.6. Antibacterial and Antifungal Assays of the Synthesized Products
3.6.1. Antibacterial Activity Assay of Monoacylglycerol
3.6.2. Antifungal Activity Assay of Monoacylglycerol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogt, R.L.; Dippold, L. Escherichia coli O157:H7 Outbreak Associated with Consumption of Ground Beef, June–July 2002. Public Health Rep. 2005, 120, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans Cell-type Switching and Functional Plasticity in the Mammalian Host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; Hamed, A.N.E.-S.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal Potential of Marine Natural Products. Eur. J. Med. Chem. 2017, 126, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Hossain, M.A.; Said, S.A. Isolation and Characterization of Antimicrobial Compound from the Stem-bark of the Traditionally Used Medicinal Plant Adenium obesum. J. Tradit. Complement. Med. 2017, 7, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Duangsrisai, S.; Choowongkomon, K.; Bessa, L.J.; Costa, P.M.; Amat, N.; Kijjoa, A. Antibacterial and EGFR-Tyrosine Kinase Inhibitory Activities of Polyhydroxylated Xanthones from Garcinia succifolia. Molecules 2014, 19, 19923–19934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Hu, Z.; Li, Q.; Huang, J.; Li, X.-N.; Zhu, H.; Liu, J.; Wang, J.; Xue, Y.; Zhang, Y. Bioassay-guided Isolation of Antibacterial Metabolites from Emericella sp. TJ29. J. Nat. Prod. 2017, 80, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S. Review of Natural Products Isolation. J. Nat. Prod. 2017, 80, 2590. [Google Scholar] [CrossRef]
- Klich, K.; Pyta, K.; Kubicka, M.M.; Ruszkowski, P.; Celewicz, L.; Gajecka, M.; Przybylski, P. Synthesis, Antibacterial, and Anticancer Evaluation of Novel Spiramycin-like Conjugates Containing C(5) Triazole Arm. J. Med. Chem. 2016, 59, 7963–7973. [Google Scholar] [CrossRef] [PubMed]
- Gullapelli, K.; Brameshwari, G.; Ravichander, M.; Kusuma, U. Synthesis, Antibacterial and Molecular Docking Studies of New Benzimidazole Derivatives. Egypt. J. Basic Appl. Sci. 2017, 4, 303–309. [Google Scholar] [CrossRef]
- Pujol, E.; Blanco-Cabra, N.; Julian, E.; Leiva, R.; Torrents, E.; Vazquez, S. Pentafluorosulfanyl-containing Triclocarban Analogs with Potent Antimicrobial Activity. Molecules 2018, 23, 2853. [Google Scholar] [CrossRef] [PubMed]
- Concilio, S.; Sessa, L.; Petrone, A.M.; Porta, A.; Diana, R.; Iannelli, P.; Piotto, S. Structure Modification of an Active Azo-Compound as a Route to New Antimicrobial Compounds. Molecules 2017, 22, 875. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, S.; Imran, M.; Habib, H.; Shabbir, S.; Ihsan, A.; Zafar, Y.; Hafeez, F.Y. Potential of Monolaurin Based Food-grade Nano-micelles Loaded with Nisin Z for Synergistic Antimicrobial Action Against Staphylocossus aureus. LWT Food Sci. Technol. 2016, 71, 227–233. [Google Scholar] [CrossRef]
- Zhong, N.; Li, L.; Xu, X.; Cheong, L.-Z.; Li, B.; Hu, S.Q.; Zhao, X. An Efficient Binary Solvent Mixture for Monoacylglycerol Synthesis by Enzymatic Glycerolysis. J. Am. Oil Chem. Soc. 2009, 86, 783–789. [Google Scholar] [CrossRef]
- Krismundsdóttir, T.; Arnadóttir, S.G.; Bergsson, G.; Thormar, H. Development and Evaluation of Microbicidal Hydrogels Containing Monoglyceride as the Active Ingredient. J. Pharm. Sci. 1999, 88, 1011–1015. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Jumina; Siswanta, D.; Sholikhah, E.N. Synthesis and Antibacterial Activity Test of 1-Monocaprin. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 74–80. [Google Scholar]
- Nitbani, F.O.; Jumina; Siswanta, D.; Solikhah, E.N. Isolation and Antibacterial Activity Test of Lauric Acid from Crude Coconut Oil (Cocos nucifera L.). Procedia Chem. 2016, 18, 132–140. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Jumina; Siswanta, D.; Sholikhah, E.N.; Fitriastuti, D. Synthesis and Antibacterial Activity of 1-Monolaurin. Orient. J. Chem. 2018, 34, 863–867. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Limmatvapirat, C.; Nunthanid, J.; Luangtana-Anan, M.; Sriamornsak, P.; Limmatvapirat, S. Design and Characterization of Monolaurin Loaded Electrospun Shellac Nanofibers with Antimicrobial Activity? Asian J. Pharm. Sci. 2018, 13, 459–471. [Google Scholar] [CrossRef]
- Odds, F.; Brown, A.J.P.; Gow, N.A.R. Antifungal Agents: Mechanism of Action. Trends Microbiol. 2003, 6, 272–279. [Google Scholar] [CrossRef]
- Pires, E.; Garcia, J.J.; Leal-Duaso, A.; Mayoral, J.A.; Garcia-Peiro, J.I.; Velazquez, D. Optimization of the Synthesis of Glycerol Derived Monoethers from Glycidol by Means Heterogeneous Acid Catalysis. Molecules 2018, 23, 2887. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Luna, D.; Bautista, F.M.; Calero, J.; Romero, A.A.; Posadillo, A.; Sancho, E.D.; Estevez, R. Evaluation of Lipases from Wild Microbial Strains as Biocatalysts in Biodiesel Production. Separations 2018, 5, 53. [Google Scholar] [CrossRef]
- CLSI. M02-A12 Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard. Available online: https://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjTovKP__PeAhVMwI8KHWE4DesQFjAAegQIABAC&url=https%3A%2F%2Fclsi.org%2Fmedia%2F1631%2Fm02a12_sample.pdf&usg=AOvVaw3agAJ-rlOnY_ldkG-bfPJp (accessed on 27 November 2018).
Sample Availability: Samples of the all synthesized products are available from the authors. |

| Sample | Inhibition Zone’s Average (mm) | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| 0.50% 1-monomyristin | 1.5 | 10.3 | - |
| 1.00% 1-monomyristin | 1.1 | 5.7 | 3.5 |
| 5.00% 1-monomyristin | 4.3 | 5.9 | 3.6 |
| 10.0% 1-monomyristin | - | 9.1 | 2.4 |
| 15.0% 1-monomyristin | 6.0 | 18.9 | 4.1 |
| 0.25% 2-monomyristin | 34.0 | 23.0 | - |
| 0.50% 2-monomyristin | 29.5 | 20.0 | - |
| 1.00% 2-monomyristin | 22.0 | 13.0 | - |
| 5.00% 2-monomyristin | 21.0 | 13.0 | - |
| 10.0% 2-monomyristin | 11.0 | 5.0 | - |
| 0.25% 2-monopalmitin | - | - | - |
| 0.50% 2-monopalmitin | - | - | - |
| 1.00% 2-monopalmitin | - | - | - |
| 5.00% 2-monopalmitin | - | - | - |
| 10.0% 2-monopalmitin | - | - | - |
| Positive control | 12.5 | 6.6 | 6.8 |
| Negative control | - | - | - |
| Sample | Inhibition Zone Average (mm) | |
|---|---|---|
| B. subtilis | A. actinomycetemcomitans | |
| 0.50% 1-monomyristin | 2.4 | 1.2 |
| 1.00% 1-monomyristin | 3.6 | 1.9 |
| 5.00% 1-monomyristin | 5.7 | 3.6 |
| 10.0% 1-monomyristin | 9.2 | 7.9 |
| 15.0% 1-monomyristin | 12.7 | 10.4 |
| Positive control | 16.3 | 5.5 |
| Negative control | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumina; Nurmala, A.; Fitria, A.; Pranowo, D.; Sholikhah, E.N.; Kurniawan, Y.S.; Kuswandi, B. Monomyristin and Monopalmitin Derivatives: Synthesis and Evaluation as Potential Antibacterial and Antifungal Agents. Molecules 2018, 23, 3141. https://doi.org/10.3390/molecules23123141
Jumina, Nurmala A, Fitria A, Pranowo D, Sholikhah EN, Kurniawan YS, Kuswandi B. Monomyristin and Monopalmitin Derivatives: Synthesis and Evaluation as Potential Antibacterial and Antifungal Agents. Molecules. 2018; 23(12):3141. https://doi.org/10.3390/molecules23123141
Chicago/Turabian StyleJumina, Asma Nurmala, Anggit Fitria, Deni Pranowo, Eti Nurwening Sholikhah, Yehezkiel Steven Kurniawan, and Bambang Kuswandi. 2018. "Monomyristin and Monopalmitin Derivatives: Synthesis and Evaluation as Potential Antibacterial and Antifungal Agents" Molecules 23, no. 12: 3141. https://doi.org/10.3390/molecules23123141