De Novo Biosynthesis of p-Coumaric Acid in E. coli with a trans-Cinnamic Acid 4-Hydroxylase from the Amaryllidaceae Plant Lycoris aurea
Abstract
:1. Introduction
2. Results and Discussion
2.1. C4H Homology of L. aurea Transcriptome
2.2. Cloning of Full-length C4H Genes in L. aurea
2.3. Subcellular Localization of LauC4H
2.4. Functional Identification of LauC4H
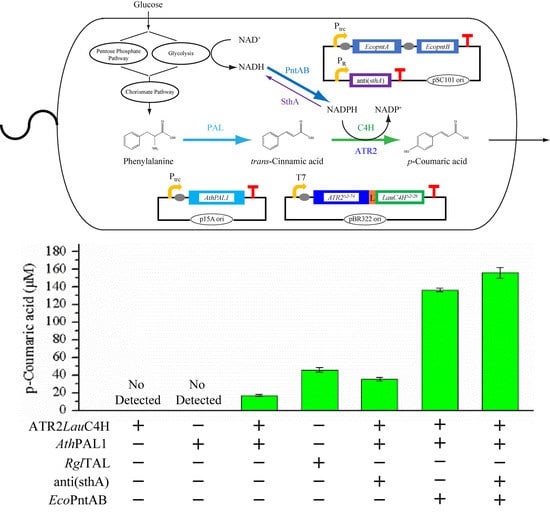
2.5. p-Coumaric Acid De Novo Biosynthesis Using LauC4H in E. coli
2.6. p-Coumaric Acid Production Improved by Intracellular NADPH Regulation
3. Materials and Methods
3.1. Plant Materials and Chemicals
3.2. RNA Extraction and Isolation of LauC4H Genes
3.3. Sequence Aanalysis of LauC4H
3.4. Subcellular Localization Analysis of LauC4H
3.5. Heterologous Expression of LauC4H in Escherichia coli
3.6. Bioconversion of LauC4H with trans-Cinnamic Acid
3.7. p-Coumaric Acid De Novo Biosynthesis in E. coli
3.8. Intracellular NADPH Regulation
3.9. Protein Detection
3.10. High Performance Liquid Chromatography (HPLC) Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A




| Name | Sequence (5′ to 3′) |
|---|---|
| RT-PCR | |
| RT-LauC4H-PF | AAGGGAAGCTTGATACCACTGAGAA |
| RT-LauC4H-PR | GACAAATTAGAACAGTCTAGGCTTGGC |
| RT-LauTIP41-PF | GCAACCATCCAAAGTTTAACTGCT |
| RT-LauTIP41-PR | AATGTGCAAGCAGGGCTAGTAA |
| cDNA cloning | |
| LauC4H-ORF-PF | ATCCTCCTCCTCCGACGAAATG |
| LauC4H-ORF-PR | AGAGTACATTGCATGGGTAATAAGGAG |
| LauC4H-5′RACE-PR | GAACTCGACCCCTTGTGTGTGC |
| LauC4H-3′RACE-PF | GGCTATGACATCCCCGCTGA |
| Subcellular localization | |
| pAN580-LauC4H-PF | AGGACCGGTCCCGGGGGATCCATGGATCTCATTCTACTAGAAAAGTCACTC |
| EGFP-LauC4H-PR | TCCTCGCCCTTGCTCACCATGAACAGTCTAGGCTTGGCCACAATG |
| EGFP-LauC4H(N28)-PR | TCCTCGCCCTTGCTCACCATCCCGCGGAGTTTGGATATTATGAT |
| pAN580-LauC4H(ΔN28)-PF | AGGACCGGTCCCGGGGGATCCATGAAGAAGCTTAAGCTCCCCCCGG |
| Functional expression | |
| 29aNdeI-LauC4H(ΔN28)-PF | AACTTTAAGAAGGAGATATACATATGAAGAAGCTTAAGCTCCCCCCGG |
| 29aXhoI-LauC4H-PR | AGTGGTGGTGGTGGTGGTGCTCGAGTTAGAACAGTCTAGGCTTGGCCACAATG |
| 29aNdeI-ATR2(ΔN74)-PF | AACTTTAAGAAGGAGATATACATATGTCCGGTTCTGGGAATTCAAAA |
| 29aXhoI-ATR2-PR | AGTGGTGGTGGTGGTGGTGCTCGAGTGAGTGTGTGGCTTCAATAGTTTCG |
| LauC4H(ΔN28)ATR2-PR | CCGGGGGGAGCTTAAGCTTCTTCATACCAGAACCAGAAGAGGTAGAACCCCATACATCTCTAAGATATCTTCCACTCGTTTG |
| De novo biosynthesis | |
| 184-trc-lacO-PF | GCCTTGCGTATAATATTTGCCCATGGTTGACAATTAATCATCCGGCTCGTATAATGTGTGGAATTGTGAGCGG |
| BBa_B0034-lacO-PR | CATATGTATTTCTCCTCTTTAGATCTGGAATTGTTATCCGCTCACAATTCCACACATTATAC |
| BBa_B0034-BBa_B0015-PF | ATCTAAAGAGGAGAAATACATATGCTCGAGTAACCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTTCGTTTTATCTGTTGTTTGTCGGTGAACGCTCTCTACTA |
| 184-BBa_B0015-PR | GCCCGCCTGATGAATGCTCATCCGGAATTCTATAAACGCAGAAAGGCCCACCCGAAGGTGAGCCAGTGTGACTCTAGTAGAGAGCGTTCACCGACAAA |
| BBa_B0034-AthPAL1-PF | ATCTAAAGAGGAGAAATACATATGGAGATTAACGGGGCACACAA |
| BBa_B0015-AthPAL1-PR | TTATTTGATGCCTGGTTACTCGAGACTTTATGGTAAGAAAAAAACAGAGGAC |
| BBa_B0034-RglPAL/TAL-PF | ATCTAAAGAGGAGAAATACATATGGCGCCGCGTCCGACCAGCCA |
| BBa_B0015-RglPAL/TAL-PR | TTATTTGATGCCTGGTTACTCGAGTTACGCCAGCATTTTCAGCAGA |
| NADPH regulation | |
| BBa_R0051-MicC-PF | TATTTTACCTCTGGCGGTGATAATGGTTGCATGCATTTTCTGTTGGGCCATTGCATTGC |
| BBa_B0015-MicC-PR | CGTTTTATTTGATGCCTGGCTCGAGAAAAAAAGCCCGGACGACTGTTC |
| BBa_B0015-PF | GCCAGGCATCAAATAAAACG |
| BBa_B0015-PR | TATAAACGCAGAAAGGCCCAC |
| pCL-BBa_R0051-PF | ACCCGTCTTACTGTCGGGAATTCTAACACCGTGCGTGTTGACTATTTTACCTCTGGCGGTGAT |
| pCL-BBa_B0015-PR | ACGTTGTAAAACGACGGCCAGTGGTACCTATAAACGCAGAAAGGCCCAC |
| anti(sthA)-PF | CCTCTGGCGGTGATAATGGTTGCATCGTAATCGTAGGAATGTGGCAT |
| anti(sthA)-PR | ATGCAATGGCCCAACAGAAAATGCCACATTCCTACGATTACGAT |
| pCL-T7-pntA-PF | GGGCCTTTCTGCGTTTATAGGTACCAAATTAATACGACTCACTATAGGGGAATCTAGAGGGAATATCATGCGAATTGGCATACC |
| pCL-BBa_B1006-pntB-PR | TTGTAAAACGACGGCCAGTGGATCCAAAAAAAACCCCGCCCTGTCAGGGGCGGGGTTTTTTTTTAAGCTTGGGTTACAGAGCTTTCAGGATTGC |
References
- Kaneko, T.; Thi, T.H.; Shi, D.J.; Akashi, M. Environmentally degradable, high-performance thermoplastics from phenolic phytomonomers. Nat Mater. 2006, 5, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Bisson, J.-F.; Skinner, M. p-Coumaric acid activates the GABA-A receptor in vitro and is orally anxiolytic in vivo. Phytother Res. 2014, 28, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, D.; Rasool, M. p-Coumaric acid, a common dietary polyphenol, protects cadmium chloride-induced nephrotoxicity in rats. Ren. Fail. 2014, 36, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Lee, N.H.; Hyun, C.G.; Shin, D.B. Differential effects of methoxylated p-coumaric acids on melanoma in B16/F10 cells. Prev. Nutr. Food Sci. 2015, 20, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sakamula, R.; Thong-Asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lin, Y.; Yan, Y. Caffeic acid production enhancement by engineering a phenylalanine over-producing Escherichia coli strain. Biotechnol. Bioeng. 2013, 110, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Katsuyama, Y.; Danyao, D.; Kahar, P.; Nakamura-Tsuruta, S.; Teramura, H.; Wakai, K.; Yoshihara, K.; Minami, H.; Ogino, C.; et al. Caffeic acid production by simultaneous saccharification and fermentation of kraft pulp using recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2017, 101, 5279–5290. [Google Scholar] [CrossRef]
- Fowler, Z.L.; Koffas, M.A. Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808. [Google Scholar] [CrossRef]
- Zhao, S.; Jones, J.A.; Lachance, D.M.; Bhan, N.; Khalidi, O.; Venkataraman, S.; Wang, Z.; Koffas, M.A. Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab. Eng. 2015, 28, 43–53. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, J.; Koffas, M.A.G. Production of anthocyanins in metabolically engineered microorganisms: Current status and perspectives. Synth. Syst. Biotechnol. 2017, 2, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Storme, V.; Vanholme, B.; Sundin, L.; Christensen, J.H.; Goeminne, G.; Halpin, C.; Rohde, A.; Morreel, K.; Boerjan, W. A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell 2012, 24, 3506–3529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achnine, L.; Blancaflor, E.B.; Rasmussen, S.; Dixon, R.A. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 2004, 16, 3098–3109. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, J.; Hamberger, B.; Million-Rousseau, R.; Werck-Reichhart, D. Cytochromes P450 in phenolic metabolism. Phytochem. Rev. 2006, 5, 239–270. [Google Scholar] [CrossRef]
- Vannelli, T.; Xue, Z.; Breinig, S.; Qi, W.W.; Sariaslani, F.S. Functional expression in Escherichia coli of the tyrosine-inducible tyrosine ammonia-lyase enzyme from yeast Trichosporon cutaneum for production of p-hydroxycinnamic acid. Enzyme Microb. Technol. 2007, 41, 413–422. [Google Scholar] [CrossRef]
- Jendresen, C.B.; Stahlhut, S.G.; Li, M.; Gaspar, P.; Siedler, S.; Förster, J.; Maury, J.; Borodina, I.; Nielsen, A.T. Highly active and specific tyrosine ammonia-lyases from diverse origins enable enhanced production of aromatic compounds in bacteria and Saccharomyces cerevisiae. Appl. Environm. Microbiol. 2015, 81, 4458–4476. [Google Scholar] [CrossRef]
- Vannelli, T.; Qi, W.W.; Sweigard, J.; Gatenby, A.A.; Sariaslani, F.S. Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab. Eng. 2007, 9, 142–151. [Google Scholar] [CrossRef]
- Vargas-Tah, A.; Martínez, L.M.; Hernández-Chávez, G.; Rocha, M.; Martínez, A.; Bolívar, F.; Gosset, G. Production of cinnamic and p-hydroxycinnamic acid from sugar mixtures with engineered Escherichia coli. Microb. Cell Fact. 2015, 14, 6. [Google Scholar] [CrossRef]
- Trotman, R.J.; Camp, C.E.; Ben-Bassat, A.; DiCosimo, R.; Huang, L.; Crum, G.A.; Sariaslani, F.S.; Haynie, S.L. Calcium alginate bead immobilization of cells containing tyrosine ammonia lyase activity for use in the production of p-hydroxycinnamic acid. Biotechnol. Prog. 2007, 23, 638–644. [Google Scholar] [CrossRef]
- Rodriguez, A.; Kildegaard, K.R.; Li, M.; Borodina, I.; Nielsen, J. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 2015, 31, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Kawai, Y.; Noda, S.; Ogino, C.; Takeshima, Y.; Okai, N.; Tanaka, T.; Kondo, A. p-Hydroxycinnamic acid production directly from cellulose using endoglucanase- and tyrosine ammonia lyase-expressing Streptomyces lividans. Microb. Cell Fact. 2013, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, K.; Westerhof, R.G.M.; Ballerstedt, H.; Bont, J.A.M.D.; Wery, J. Optimization of the solvent-tolerant Pseudomonas putida S12 as host for the production of p-coumarate from glucose. Appl. Microb. Biotechnol. 2007, 74, 617. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.; Yan, Y.; Koffas, M.A.G. Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab. Eng. 2006, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.; Koffas, M.A. Engineering of artificial plant cytochrome P450 enzymes for synthesis of isoflavones by Escherichia coli. Appl. Environm. Microb. 2007, 73, 7246–7251. [Google Scholar] [CrossRef]
- Wang, R.; Xu, S.; Jiang, Y.; Jiang, J.; Li, X.; Liang, L.; He, J.; Peng, F.; Xia, B. De novo sequence assembly and characterization of Lycoris aurea transcriptome using GS FLX Titanium platform of 454 pyrosequencing. PLoS ONE 2013, 8, e60449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, S.; Han, X.; Wang, R.; He, J.; Xia, B.; Wang, R. Investigation of nuclear DNA contents of Lycoris species (Amaryllidaceae) with different chromosome number by flow cytometry. Pak. J. Botany. 2017, 49, 2197–2200. [Google Scholar]
- Kilgore, M.B.; Augustin, M.M.; May, G.D.; Crow, J.A.; Kutchan, T.M. CYP96T1 of Narcissus sp. aff. pseudonarcissus catalyzes formation of the para-para’ C–C phenol couple in the Amaryllidaceae alkaloids. Front Plant Sci. 2016, 7, 225. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Augustin, M.M.; Starks, C.M.; O’Neil-Johnson, M.; May, G.D.; Crow, J.A.; Kutchan, T.M. Cloning and characterization of a norbelladine 4′-O-methyltransferase involved in the biosynthesis of the Alzheimer’s drug galanthamine in Narcissus sp. aff. pseudonarcissus. PLoS ONE 2014, 9, e103223. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Nielsen, H.; von Heijne, G.; Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004, 340, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mignotte, C.; Kazmaier, M.; Delorme, F.; Pompon, D. Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J. Biol. Chem. 1997, 272, 19176–19186. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.K.; Ehlting, J.; Douglas, C.J. Cloning, functional expression, and subcellular localization of multiple NADPH-cytochrome P450 reductases from hybrid poplar. Plant Physiol. 2002, 130, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Sato, K.; Suhara, K.; Sakaguchi, M.; Mihara, K.; Omura, T. Importance of the proline-rich region following signal-anchor sequence in the formation of correct conformation of microsomal cytochrome P-450s. J. Biochem. 1993, 114, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Na, D.; Yoo, S.M.; Chung, H.; Park, H.; Park, J.H.; Lee, S.Y. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat. Biotechnol. 2013, 31, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U.; Canonaco, F.; Heri, S.; Perrenoud, A.; Fischer, E. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in nadph metabolism of Escherichia coli. J. Biol. Chem. 2004, 279, 6613. [Google Scholar] [CrossRef]
- Ma, R.; Xu, S.; Zhao, Y.; Xia, B.; Wang, R. Selection and validation of appropriate reference genes for quantitative real-time PCR analysis of gene expression in Lycoris aurea. Front Plant Sci. 2016, 7, 536. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Chen, S.; Tao, L.; Zeng, L.; Vega-Sanchez, M.E.; Umemura, K.; Wang, G.L. A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef]
- Fasan, R.; Crook, N.C.; Peters, M.W.; Meinhold, P.; Buelter, T.; Landwehr, M.; Cirino, P.C.; Arnold, F.H. Improved product-per-glucose yields in p450-dependent propane biotransformations using engineered Escherichia coli. Biotechnol. Bioeng. 2011, 108, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Cohen, S.N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 1978, 134, 1141–1156. [Google Scholar] [PubMed]
- Rose, R.E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988, 16, 355. [Google Scholar] [CrossRef]
- Lerner, C.G.; Inouye, M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990, 18, 4631. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Zhang, X.; Yang, P.; Liang, Q.; Qi, Q. A novel whole-phase succinate fermentation strategy with high volumetric productivity in engineered Escherichia coli. Biores. Technol. 2013, 149, 333–340. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Strain | Biomass (OD600) | Glucose Utilization (g/L) | trans-Cinnamic Acid (μM) | p-Coumaric Acid (μM) |
|---|---|---|---|---|
| Ec/LauC4H | 5.56 ± 0.72 | 13.50 ± 0.72 | ND 2 | ND 2 |
| Ec/AthPAL | 8.92 ± 0.85 | 24.50 ± 0.65 | 241.32 ± 13.24 | ND 2 |
| Ec/RglPAL(TAl) | 9.73 ± 1.24 | 25.08 ± 0.75 | 91.94 ± 6.32 | 46.09 ± 2.96 |
| Ec/LauC4H-AthPAL | 7.66 ± 0.83 | 24.78 ± 1.26 | 342.82 ± 15.80 | 17.22 ± 1.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, J.; Qian, B.; Cheng, L.; Xu, S.; Wang, R. De Novo Biosynthesis of p-Coumaric Acid in E. coli with a trans-Cinnamic Acid 4-Hydroxylase from the Amaryllidaceae Plant Lycoris aurea. Molecules 2018, 23, 3185. https://doi.org/10.3390/molecules23123185
Li Y, Li J, Qian B, Cheng L, Xu S, Wang R. De Novo Biosynthesis of p-Coumaric Acid in E. coli with a trans-Cinnamic Acid 4-Hydroxylase from the Amaryllidaceae Plant Lycoris aurea. Molecules. 2018; 23(12):3185. https://doi.org/10.3390/molecules23123185
Chicago/Turabian StyleLi, Yikui, Jie Li, Binbin Qian, Li Cheng, Sheng Xu, and Ren Wang. 2018. "De Novo Biosynthesis of p-Coumaric Acid in E. coli with a trans-Cinnamic Acid 4-Hydroxylase from the Amaryllidaceae Plant Lycoris aurea" Molecules 23, no. 12: 3185. https://doi.org/10.3390/molecules23123185