Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview
Abstract
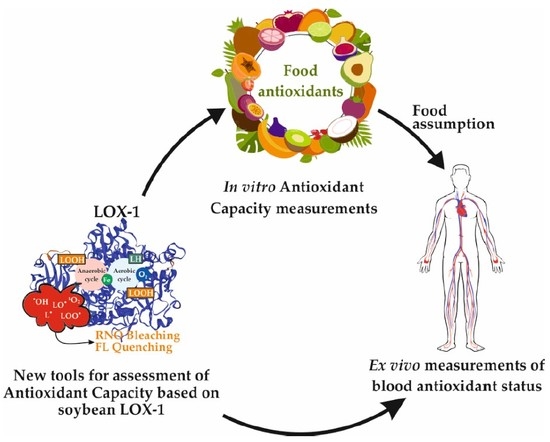
:1. Introduction
2. Methods Based on the Use of the Soybean Lipoxygenase-1 Isoenzyme: Lipoxygenase/4-Nitroso-N,N-Dimethylaniline (LOX/RNO) and Lipoxygenase–Fluorescein (LOX-FL) Assays
2.1. Aerobic and Anaerobic Reactions Catalyzed by Soybean Lipoxygenase (LOX)-1 Isoform and Involvement of LOX-1-Mediated Reactions in RNO Bleaching and FL Quenching
2.2. LOX/RNO and LOX–FL Reactions: Main Kinetic Properties
2.3. Inhibition of LOX/RNO and LOX–FL Reactions by Pure Antioxidant Compounds
2.4. Suitability of the LOX-1-Based Assays to Assess AC of Plant Food Extracts and Blood Samples
2.4.1. Assessment of AC of Plant Food Extracts
2.4.2. Assessment of AC of Blood Samples
2.5. Evaluation of Synergistic Effects Among Antioxidants Using LOX-1-Based Assays
3. Evaluation of Human Blood Antioxidant Status after Food Intake
3.1. Antioxidant/Oxidant Balance (AOB) Approach and its Application in Short-Term Studies
3.2. AOB Approach in Long-Term Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; German, J.B. Antioxidants in foods and health: Problems and fallacies in the field. J. Sci. Food Agric. 2006, 86, 1999–2001. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH. Radical in Alcoholic Solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M. Inhibition of the bleaching of the carotenoid crocin a rapid test for quantifying antioxidant activity. Biochim. Biophys. Acta 1984, 796, 312–319. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Triantis, T.; Yannakopoulou, E.; Nikokavoura, A.; Dimotikali, D. Comparative studies on the antioxidant activity of aqueous extracts of olive oils and seed oils using chemiluminescence. Anal. Chim. Acta 2003, 494, 41–47. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanism of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Bumke-Vogt, C.; Osterhoff, M.A.; Borchert, A.; Guzman-Perez, V.; Sarem, Z.; Birkenfeld, A.L.; Bähr, V.; Pfeiffer, A.F.H. The Flavones Apigenin and Luteolin Induce FOXO1 Translocation but Inhibit Gluconeogenic and Lipogenic Gene Expression in Human Cells. PLoS ONE 2014, 9, e104321. [Google Scholar] [CrossRef] [PubMed]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; D’Hellencourt, C.L.; Gonthier, M.-P.; Da Silva, C.R. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismut. J. Inflamm. 2015, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Laus, M.N.; Tozzi, D.; Fogliano, V.; Soccio, M.; Flagella, Z. New tool to evaluate a comprehensive antioxidant activity in food extracts: Bleaching of 4-nitroso-N,N-dimethylaniline catalyzed by soybean lipoxygenase-1. J. Agric. Food Chem. 2009, 57, 9682–9692. [Google Scholar] [CrossRef] [PubMed]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Pastore, D. The soybean lipoxygenase-fluorescein reaction may be used to assess antioxidant capacity of phytochemicals and serum. Anal. Methods 2016, 8, 4354–4362. [Google Scholar] [CrossRef] [Green Version]
- Pastore, D.; Trono, D.; Padalino, L.; Di Fonzo, N.; Passarella, S. p-Nitrosodimethylaniline (RNO) bleaching by soybean lipoxygenase-1. Biochemical characterization and coupling with oxodiene formation. Plant Physiol. Biochem. 2000, 38, 845–852. [Google Scholar] [CrossRef]
- Laus, M.N.; Tozzi, D.; Soccio, M.; Fratianni, A.; Panfili, G.; Pastore, D. Dissection of antioxidant activity of durum wheat (Triticum durum Desf.) grains as evaluated by the new LOX/RNO method. J. Cereal Sci. 2012, 56, 214–222. [Google Scholar] [CrossRef]
- Laus, M.N.; Gagliardi, A.; Soccio, M.; Flagella, Z.; Pastore, D. Antioxidant Activity of Free and Bound Compounds in Quinoa (Chenopodium quinoa Willd.) Seeds in Comparison with Durum Wheat and Emmer. J. Food Sci. 2012, 77, C1150–C1155. [Google Scholar] [CrossRef]
- Laus, M.N.; Denoth, F.; Ciardi, M.; Giorgetti, L.; Pucci, L.; Sacco, R.; Pastore, D.; Longo, V. Antioxidant-rich food supplement Lisosan G induces reversion of hepatic steatosis. Med. Weter. 2013, 69, 235–240. [Google Scholar]
- Laus, M.N.; Soccio, M.; Pastore, D. Evaluation of synergistic interactions of antioxidants from plant foods by a new method using soybean lipoxygenase. J. Food Nutr. Res. 2013, 52, 256–260. [Google Scholar]
- Laus, M.N.; Soccio, M.; Giovannini, D.; Caboni, E.; Quacquarelli, I.; Maltoni, M.L.; Scossa, F.; Condello, E.; Pastore, D. Assessment of antioxidant activity of carotenoid-enriched extracts from peach fruits using the new LOX/RNO method. Adv. Hortic. Sci. 2015, 29, 75–83. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Laus, M.N.; Soccio, M.; Alfarano, M.; Pasqualone, A.; Lenucci, M.S.; Di Miceli, G.; Pastore, D. Different effectiveness of two pastas supplemented with either lipophilic or hydrophilic/phenolic antioxidants in affecting serum as evaluated by the novel Antioxidant/Oxidant Balance approach. Food Chem. 2017, 221, 278–288. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Dalfino, G.; Panunzio, M.F.; Pastore, D. Antioxidant/Oxidant Balance as a novel approach to evaluate the effect on serum of long-term intake of plant antioxidant-rich foods. J. Funct. Foods 2018, 40, 778–784. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzyme Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef]
- Schneider, C.; Pratt, D.A.; Porter, N.A.; Brash, A.R. Control of Oxygenation in Lipoxygenase and Cyclooxygenase Catalysis. Chem. Biol. 2007, 14, 473–488. [Google Scholar] [CrossRef] [Green Version]
- Liavonchanka, A.; Feussner, I. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Physiol. 2006, 163, 348–357. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant lipoxygenase: Structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Hildebrand, D.F. Lipoxygenases. Physiol. Plant. 1989, 76, 249–253. [Google Scholar] [CrossRef]
- Yamamoto, S. Mammalian lipoxygenases: Molecular structures and functions. Biochim. Biophys. Acta 1992, 1128, 117–131. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef]
- Samuelsson, B.; Dahlén, S.-E.; Lindgren, J.Å.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Sigal, E.; Laughton, C.W.; Mulkins, M.A. Oxidation, Lipoxygenase, and Atherogenesis. Ann. NY Acad. Sci. 1994, 714, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.C.; Pérez, A.G.; Olías, J.M. Lipoxygenase in the plant kingdom. I. Properties. Grasas Y Aceites 1992, 43, 231–239. [Google Scholar] [CrossRef]
- Garssen, G.J.; Vliegenthart, J.F.; Boldingh, J. An anaerobic reaction between lipoxygenase, linoleic acid and its hydroperoxides. Biochem. J. 1971, 122, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanofsky, J.R.; Axelrod, B. Singlet oxygen production by soybean lipoxygenase isozymes. J. Biol. Chem. 1986, 261, 1099–1104. [Google Scholar]
- Kraljić, I.; Mohsni, S.E. A new method for the detection of singlet oxygen in aqueous solutions. Photochem. Photobiol. 1978, 28, 577–581. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Akhilender Naidu, K. Antioxidant activity of selected Indian spices. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 107–110. [Google Scholar] [CrossRef]
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Chaillou, L.L.; Nazareno, M.A. New method to determine antioxidant activity of polyphenols. J. Agric. Food Chem. 2006, 54, 8397–8402. [Google Scholar] [CrossRef] [PubMed]
- Lomnitski, L.; Bar-Natan, R.; Sklan, D.; Grossman, S. The interaction between β-carotene and lipoxygenase in plant and animal systems. Biochim. Biophys. Acta 1993, 1167, 331–338. [Google Scholar] [CrossRef]
- Barimalaa, I.S.; Gordon, M.H. Cooxidation of β-Carotene by Soybean Lipoxygenase. J. Agric. Food Chem. 1988, 36, 685–687. [Google Scholar] [CrossRef]
- Gagliardi, A.; Laus, M.N.; DeVita, P.; Flagella, Z.; Pastore, D. Antioxidant activity in wheat grains with different ploidy levels. Ital. J. Agron. 2008, 3, 419–421. [Google Scholar]
- Laus, M.N.; Soccio, M.; Flagella, Z.; Pastore, D. Antioxidant activity of free versus bound compounds in seeds of different cereal species. In Proceedings of the 12th Congress of the European Society for Agronomy, Helsinki, Finland, 20–24 August 2012; pp. 486–487. [Google Scholar]
- Flagella, Z.; Tozzi, D.; Soccio, M.; Tarantino, E.; Pastore, D. Grain antioxidant activity in different herbaceous crop species. Fragm. Agron. 2006, 11, 753–754. [Google Scholar]
- Pastore, D.; Tozzi, D.; Laus, M.N.; Soccio, M.; Fogliano, V.; Flagella, Z. Un metodo innovativo per la determinazione dell’attività antiossidante totale in matrici alimentari basato sull’utilizzo dell’enzima Lipossigenasi. In Proceedings of the XXXIV Congress of the Italian Society of Human Nutrition, Riccione (RM), Italy, 8–10 November 2006; p. 87. [Google Scholar]
- Laus, M.N.; Tozzi, D.; Gagliardi, A.; Bimbo, F.; Flagella, Z.; Pastore, D. Messa a punto di un nuovo metodo di determinazione dell’attività antiossidante totale in matrici alimentari basato sulla reazione di bleaching della p-nitrosodimetilanilina (RNO) catalizzata dalla Lipossigenasi di soia. In Proceedings of the V Congress of the Italian Association of Agricultural Science Societies, Foggia, Italy, 10–12 December 2007; pp. 112–113. [Google Scholar]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Gervasi, P.G.; Lubrano, V. Cisplatin induced toxicity in rat tissues: The protective effect of Lisosan G. Food Chem. Toxicol. 2011, 49, 233–237. [Google Scholar] [CrossRef]
- Frassinetti, S.; Della Croce, C.M.; Caltavuturo, L.; Longo, V. Antimutagenic and antioxidant activity of Lisosan G in Saccharomyces cerevisiae. Food Chem. 2012, 135, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- La Marca, M.; Beffy, P.; Pugliese, A.; Longo, V. Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLoS ONE 2013, 8, e83538. [Google Scholar] [CrossRef]
- Lucchesi, D.; Russo, R.; Gabriele, M.; Longo, V.; Del Prato, S.; Penno, G.; Pucci, L. Grain and Bean Lysates Improve Function of Endothelial Progenitor Cells from Human Peripheral Blood: Involvement of the Endogenous Antioxidant Defenses. PLoS ONE 2014, 9, e109298. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Khan, I.; Yousif, A.M.; Johnson, S.K.; Gamlath, S. Acute effect of sorghum flour-containing pasta on plasma total polyphenols, antioxidant capacity and oxidative stress markers in healthy subjects: A randomised controlled trial. Clin. Nutr. 2015, 34, 415–421. [Google Scholar] [CrossRef]
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabaté, J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J. Hum. Nutr. Diet. 2009, 22, 64–71. [Google Scholar] [CrossRef]
- Fernández-Panchón, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef]
- Matthaiou, C.M.; Goutzourelas, N.; Stagos, D.; Sarafoglou, E.; Jamurtas, A.; Koulocheri, S.D.; Haroutounian, S.A.; Tsatsakis, A.M.; Kouretas, D. Pomegranate juice consumption increases GSH levels and reduces lipid and protein oxidation in human blood. Food Chem. Toxicol. 2014, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Pujadas, G.; La Sala, L.; Testa, R.; Bonfigli, A.R.; Genovese, S. Hyperglycemia following recovery from hypoglycemia worsens endothelial damage and thrombosis activation in type 1 diabetes and in healthy controls. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Khor, A.; Grant, R.; Tung, C.; Guest, J.; Pope, B.; Morris, M.; Bilgin, A. Postprandial oxidative stress is increased after a phytonutrient-poor food but not after a kilojoule-matched phytonutrient-rich food. Nutr. Res. 2014, 34, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Laus, M.N.; Soccio, M.; Alfarano, M.; Pasqualone, A.; Lenucci, M.S.; Di Miceli, G.; Pastore, D. Serum antioxidant capacity and peroxide level of seven healthy subjects after consumption of different foods. Data Br. 2016, 9, 818–822. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, M.G.; Soccio, M.; Pastore, D.; Albenzio, M.; Sevi, A.; Caroprese, M. Antioxidant/oxidant balance: Application as a biomarker of the antioxidant status in plasma of ewes fed seaweed Ascophyllum nodosum and flaxseed under high ambient temperature. Small Rumin. Res. 2019, 170, 102–108. [Google Scholar] [CrossRef]
- Szponder, T.; Wessely-Szponder, J.; Świeca, M.; Smolira, A.; Gruszecki, T. The combined use of ozone therapy and autologous platelet-rich plasma as an alternative approach to foot rot treatment for sheep. A preliminary study. Small Rumin. Res. 2017, 156, 50–56. [Google Scholar] [CrossRef]






| Assay | Main Mechanism | Oxidant | Probe | Detection | Ref. |
|---|---|---|---|---|---|
| ORAC | HAT | ROO∙ | FL | Fluorescence | [1,7] |
| DPPH | SET | DPPH∙ | DPPH∙ | Absorbance | [8,9] |
| FRAP | SET | Fe3+ | [Fe(TPTZ)2]2+ | Absorbance | [10] |
| TEAC | SET | ABTS∙+ | ABTS+ | Absorbance | [11,12] |
| HORAC | HAT | HO∙ | FL | Fluorescence | [13] |
| TRAP | HAT | ROO∙ | β-PE | Fluorescence | [14,15] |
| CUPRAC | SET | Cu2+ | Neocuproine | Absorbance | [16] |
| Total Phenolic Assay | SET | FCR | FCR | Absorbance | [17] |
| Crocin Bleaching | HAT | ROO∙ | Crocin | Absorbance | [18] |
| Chemiluminescence | HAT | H2O2 | Luminol | Fluorescence | [19] |
| Compound | Ki a or IC50 b | Antioxidant/Trolox c |
|---|---|---|
| LOX/RNO Reaction | ||
| Trolox | 7.0 ± 1.1 mM a,d | 1.00 |
| Resveratrol | 1.7 ± 0.2 mM a,d | 4.57 ± 0.33 e |
| Ferulic acid | 10.7 ± 2.1 mM a,d | 0.99 ± 0.11e |
| Gallic acid | 6.7 ± 1.3 mM a,d | 0.61 ± 0.05 e |
| Apigenin | 3.4 ± 0.5 mM a,d | 1.49 ± 0.09 e |
| Catechin | 13.6 ± 1.8 mM a,d | 0.42 ± 0.03 e |
| l-Ascorbic acid | 14.0 ± 1.9 mM a,d | 0.83 ± 0.09 e |
| Glutathione | 19.7 ± 3.2 mM b,d | n.d. |
| α-tocopherol | 1.1 ± 0.1 mM a,d | 3.43 ± 0.25 e |
| β-carotene | 7.8 ± 0.9 μM b,d | n.d. |
| LOX–FL Reaction | ||
| Trolox | 5 ± 0.6 μM a; 26 ± 2 μM b,d | 1.00 |
| Albumin | 25 ± 2 μM b,d | 1.04 ± 0.08 e,f |
| Bilirubin | 7.6 ± 0.6 μM b,d | 3.43 ± 0.28 e,f |
| l-Ascorbic acid | 1360 ± 10 μM b,d | 0.02 ± 0.002 e,f |
| Uric acid | 40 ± 2 μM b,d | 0.66 ± 0.04 e,f |
| Plant Matrices and Derived Products | Extract | AC (µmol Trolox eq./g Dry Weight) | Ref. | ||
|---|---|---|---|---|---|
| LOX/RNO and/or LOX–FL a | ORAC | TEAC | |||
| Whole grains of durum wheat (Triticum durum Desf., cv. Simeto) | H | 102 ± 4.8 b | 3.00 ± 0.05 b | [28] | |
| L | 46.3 ± 1.1 b | 0.34 ± 0.01 b | |||
| FSP | 41.1 ± 9.7 b | ||||
| IBP | 1137 ± 65 b | 6.23 ± 0.12 b | |||
| Whole grains of durum wheat (Triticum durum Desf., cv. Ofanto) | H | 116 ± 9 b | 19.3 ± 2.1 | 2.98 ± 0.09 b | [25,28] |
| L | 57.3 ± 1.1 b | 2.26 ± 0.17 | 0.35 ± 0.01 b | ||
| FSP | 133 ± 44 b | ||||
| IBP | 1336 ± 44 b | 14.7 ± 2.0 | 6.00 ± 0.20 b | ||
| Whole grains of durum wheat (Triticum durum Desf., cv. Adamello) | H | 106 ± 8 | 17.3 ± 1.5 | 5.13 ± 0.40 | [29] |
| L | 73.3 ± 11.9 | 1.46 ± 0.06 | 0.24 ± 0.04 | ||
| FSP | 47.2 ± 0.8 | 3.42 ± 0.16 | 0.89 ± 0.02 | ||
| IBP | 800 ± 12 | 13.5 ± 1.5 | 6.67 ± 0.14 | ||
| Whole grains of bread wheat (Triticum aestivum L., cv. Bolero) | H | 128 ± 10 | 32.5 ± 3.5 | 5.34 ± 0.10 | [57,58] |
| L | 146 ± 14 | 15.5 ± 0.9 | 0.19 ± 0.02 | ||
| FSP | 54.0 ± 2.0 | 2.51 ± 0.11 | 0.97 ± 0.03 | ||
| IBP | 256 ± 8 | 8.28 ± 1.07 | 3.92 ± 0.21 | ||
| Whole grains of naked einkorn (Triticum monococcum L. ssp. sinskajae) | H | 115 ± 12 | 4.45 ± 0.16 | 5.61 ± 0.09 | [57,58] |
| L | 210 ± 8 | 2.83 ± 0.06 | 0.58 ± 0.02 | ||
| FSP | 74.2 ± 2.3 | 2.42 ± 0.12 | 0.73 ± 0.03 | ||
| IBP | 1081 ± 22 | 1.93 ± 0.07 | 0.63 ± 0.01 | ||
| Whole grains of hulled einkorn (Triticum monococcum L. ssp. monococcum) | H | 72.3 ± 3.8 | 3.59 ± 0.02 | 5.76 ± 0.30 | [57,58] |
| L | 148 ± 11 | 2.95 ± 0.25 | 0.40 ± 0.05 | ||
| FSP | 263 ± 6 | 1.70 ± 0.01 | 0.51 ± 0.01 | ||
| IBP | 2008 ± 38 | 12.0 ± 1.0 | 4.95 ± 0.17 | ||
| Whole grains of emmer (Triticum dicoccum Schübler, cv. Molise Colli) | H | 360 ± 65 | 54.6 ± 13.0 | 5.84 ± 0.12 | [29] |
| L | 141 ± 11 | 0.88 ± 0.03 | 0.20 ± 0.01 | ||
| FSP | 14.2 ± 0.7 | 4.25 ± 0.41 | 0.75 ± 0.04 | ||
| IBP | 1239 ± 21 | 26.0 ± 0.4 | 1.61 ± 0.09 | ||
| Whole grains of spelt (Triticum spelta L., cv. Altgold Rotkorn) | H | 256 ± 11 | 22.5 ± 1.7 | 5.23 ± 0.15 | [57,58] |
| L | 104 ± 12 | 2.27 ± 0.02 | 0.80 ± 0.02 | ||
| FSP | 186 ± 4 | 2.51 ± 0.07 | 0.66 ± 0.02 | ||
| IBP | 1764 ± 25 | 5.84 ± 0.25 | 1.50 ± 0.05 | ||
| Whole grains of finger millet (Eleusine coracana L. Gaertn.) | H | 565 ± 5 | 25 ± 0.1 | [59,60,61] | |
| Whole grains of teff (Eragrostis tef (Zucc.) Trotter) | H | 256 ± 8 | 2 ± 0.1 | [59,60,61] | |
| Whole grains of buckwheat (Fagopyrum esculentum Moench) | H | 82 ± 9.8 | 16 ± 1.2 | [60,61] | |
| Whole grains of amaranth (Amaranthus spp.) | H | 64 ± 1 | 3 ± 0.1 | [59,60,61] | |
| Saponin-free grains of quinoa (Chenopodium quinoa Willd., cv. Real) | H | 138 ± 11 | 37 ± 1 | 12.8 ± 0.5 | [29] |
| L | 130 ± 6 | 0.38 ± 0.03 | 0.33 ± 0.03 | ||
| FSP | 81 ± 4 | 5.75 ± 0.23 | 1.67 ± 0.06 | ||
| IBP | 428 ± 4 | 4.89 ± 0.15 | 3.72 ± 0.17 | ||
| Durum wheat bran | BW | 749 ± 50 (12.5 ± 0.7 a) f | 48 ± 4 f | 18.9 ± 1.3 f | |
| Lisosan G (nutritional supplement) | H | 1576 ± 427 (81.7 ± 4.7 a) | 123 ± 6 | 48 ± 3 | [26,30] |
| L | 258 ± 2 (0.56 ± 0.06 a) | 1.3 ± 0.02 | 3.7 ± 0.78 | ||
| FSP | 83 ± 2 (4.3 ± 0.4 a) | 25.6 ± 0.7 | 7.1 ± 1.2 | ||
| IBP | 1294 ± 24 (7.1 ± 0.1 a) | 56.6 ± 4.8 | 29.5 ± 2.1 | ||
| Apple (Malus domestica Borkh.) fiber | H | 333 ± 23 | 29 ± 2 | [60,61] | |
| Coffee silverskin | H | 1773 ± 108 | 27 ± 0.3 | [60,61] | |
| Durum wheat semolina pasta | H | 2.55 ± 0.09 a | 5.15 ± 0.55 | 2.29 ± 0.20 | [34] |
| L | 0.41 ± 0.04 a | 1.11 ± 0.11 | 0.14 ± 0.02 | ||
| FSP | 0.34 ± 0.02 a | 1.46 ± 0.16 | 0.20 ± 0.03 | ||
| Durum wheat semolina pasta supplemented with durum wheat bran oleoresin extract | H | 2.93 ± 0.11 a | 6.03 ± 0.49 | 2.13 ± 0.1 | [34] |
| L | 1.58 ± 0.1 a | 0.86 ± 0.11 | 0.19 ± 0.003 | ||
| FSP | 0.29 ± 0.01 a | 1.69 ± 0.19 | 0.19 ± 0.01 | ||
| Durum wheat semolina pasta supplemented with durum wheat bran water extract | H | 2.39 ± 0.1 a | 4.56 ± 0.25 | 2.34 ± 0.12 | [34] |
| L | 0.35 ± 0.02 a | 1.13 ± 0.08 | 0.15 ± 0.01 | ||
| FSP | 0.92 ± 0.03 a | 1.50 ± 0.03 | 0.29 ± 0.01 | ||
| Food-grade resveratrol (98%) from Japanese knotweed (Polygonum cuspidatum Siebold et Zucc.) root | - | 28.2 ± 0.6 c | 6.44 ± 0.71 c | [31] | |
| Food-grade quercetin (98%) from Japanese pagoda tree (Sophora japonica L.) flower buds | - | 12.7 ± 0.5 c | 5.87 ± 0.50 c | [31] | |
| Food-grade catechins (50%) from green tea (Camellia sinensis (L.) Kuntze) leaf | - | 29.8 ± 0.7 c | 3.15 ± 0.45 c | [31] | |
| Food-grade lycopene (15%) from tomato (Solanum lycopersicum L.) fruit | - | 215 ± 1.5 c | 2.45 ± 0.30 c | [31] | |
| OLIPLUS®, olive (Olea europaea L.) extract containing polyphenols (45%) | - | 24.0 ± 1.0 c | 1.69 ± 0.30 c | [31] | |
| Extra virgin olive oil (Olea europaea L., cv. Cima di Mola) | - | 4030 ± 400 d | 17.3 ± 1.8 d | 4.4 ± 0.2 d | u.d. |
| Extra virgin olive oil (Olea europaea L., cv. Coratina) | - | 2800 ± 160 d | 26.0 ± 2.7 d | 3.9 ± 0.3 d | u.d. |
| Extra virgin olive oil (Olea europaea L., cv. Peranzana) | - | 2770 ± 10 d | 14.2 ± 0.1 d | 2.01 ± 0.07 d | u.d. |
| Red wine (Vitis vinifera L., cv. Negramaro) | - | 8.6 ± 0.2 a,d | 59.8 ± 5.8 d | 24.4 ± 1.3 d | u.d. |
| Red wine (Vitis vinifera L., cv. Nero di Troia) | - | 10.7 ± 0.3 a,d | 47.1 ± 2.8 d | 36.6 ± 0.9 d | u.d. |
| Red wine (Vitis vinifera L., cv. Primitivo) | - | 8.0 ± 0.3 a,d | 47.6 ± 6.0 d | 32.6 ± 1.3 d | u.d. |
| Puree from cherry (Prunus avium L., cv. Ferrovia) fruit | FSP | 5.1 ± 0.2 a,e | 19.1 ± 0.1 e | 6.5 ± 0.1 e | [35] |
| Peach (Prunus persica L., cv. Redhaven) fruit | C | 155 ± 10 e | 0.33 ± 0.04 e | 0.082 ± 0.002 e | [32] |
| Peach (Prunus persica L., cv. Armking) fruit | C | 125 ± 10 e | 0.44 ± 0.06 e | 0.068 ± 0.001 e | [32] |
| Peach (Prunus persica L., cv. Silverking) fruit | C | 9.6 ± 3.5 e | 0.21 ± 0.04 e | 0.025 ± 0.001 e | [32] |
| Peach (Prunus persica L., cv. Caldesi 2000) fruit | C | 10.5 ± 1.2 e | 0.11 ± 0.01 e | 0.018 ± 0.0001 e | [32] |
| Peach (Prunus persica L., cv. IFF331) fruit | C | 9.8 ± 1.5 e | 0.13 ± 0.01 e | 0.020 ± 0.0001 e | [32] |
| Tomato (Solanum lycopersicum L.) fruit | H | 3.79 ± 0.84 a | 64.4 ± 10.7 | 38.3 ± 4.0 | u.d. |
| L | 11.25 ± 5.24 a | 41.6 ± 15.4 | 3.88 ± 0.87 | ||
| Juice of pomegranate (Punica granatum L.) fruit | - | 36.2 ± 5.4 a,d | 14.6 ± 3.1 d | 45.8 ± 1.5 d | u.d. |
| LOX/RNO a or LOX–FL b | ORAC | TEAC | Ref. | |
|---|---|---|---|---|
| Among Different Pure Compounds (% Change) c | ||||
| Mix of the food-grade extracts resveratrol, quercetin, OLIPLUS®, catechin and lycopene | +570 ***,a | - | +30 ** | [31] |
| Mix of ascorbic acid, Trolox, bilirubin, uric acid and albumin | +74 ***,b | +12 n.s. | - | [26] |
| Among different extracts (% change) c | ||||
| Mix of hydrophilic, lipophilic and insoluble-bound phenolic extracts from durum wheat whole flour | +108 **,a | +39 * | −32 * | [25] |
| Among phenols in the same extract (time fold change) d | ||||
| Insoluble-bound phenols from durum wheat whole flour | 410 a | 2.8 | 0.89 | [28] |
| Among human serum and free-soluble phenols (% change) c | ||||
| Mix of human blood serum and free-soluble phenolic extract from Lisosan G | +124 ***,b | +16 * | - | [26] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soccio, M.; Laus, M.N.; Flagella, Z.; Pastore, D. Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview. Molecules 2018, 23, 3244. https://doi.org/10.3390/molecules23123244
Soccio M, Laus MN, Flagella Z, Pastore D. Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview. Molecules. 2018; 23(12):3244. https://doi.org/10.3390/molecules23123244
Chicago/Turabian StyleSoccio, Mario, Maura N. Laus, Zina Flagella, and Donato Pastore. 2018. "Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products Using Soybean Lipoxygenase-Based Methods. An Overview" Molecules 23, no. 12: 3244. https://doi.org/10.3390/molecules23123244