Combination of Dry Milling and Separation Processes with Anaerobic Digestion of Olive Mill Solid Waste: Methane Production and Energy Efficiency
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Dry Fractionation on OP Biochemical Composition
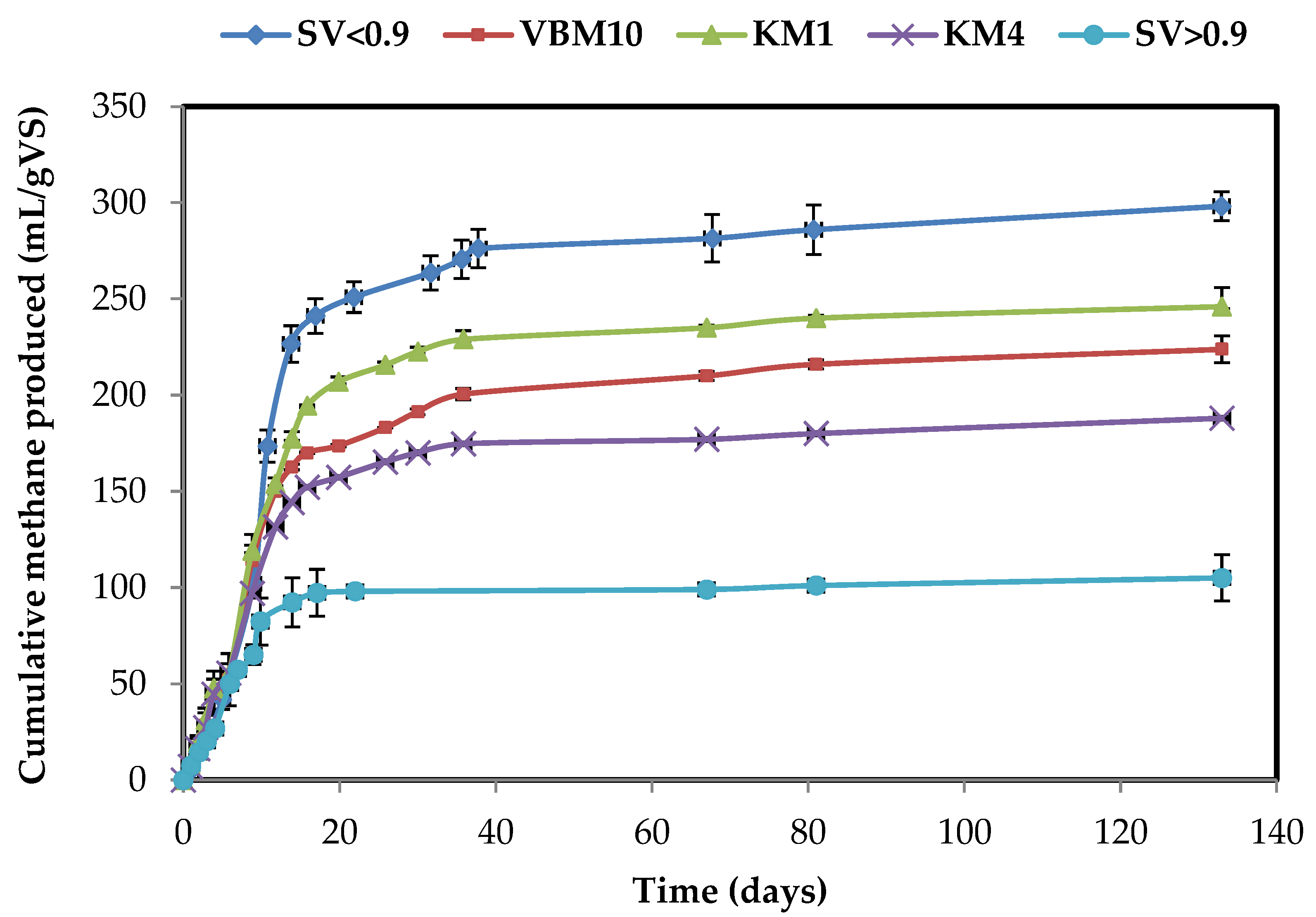
2.2. Effects of Milling and Dry Fractionation Processes on Methane Production
2.3. Pretreatment Energy Efficiency
3. Materials and Methods
3.1. Biomass and Inoculum
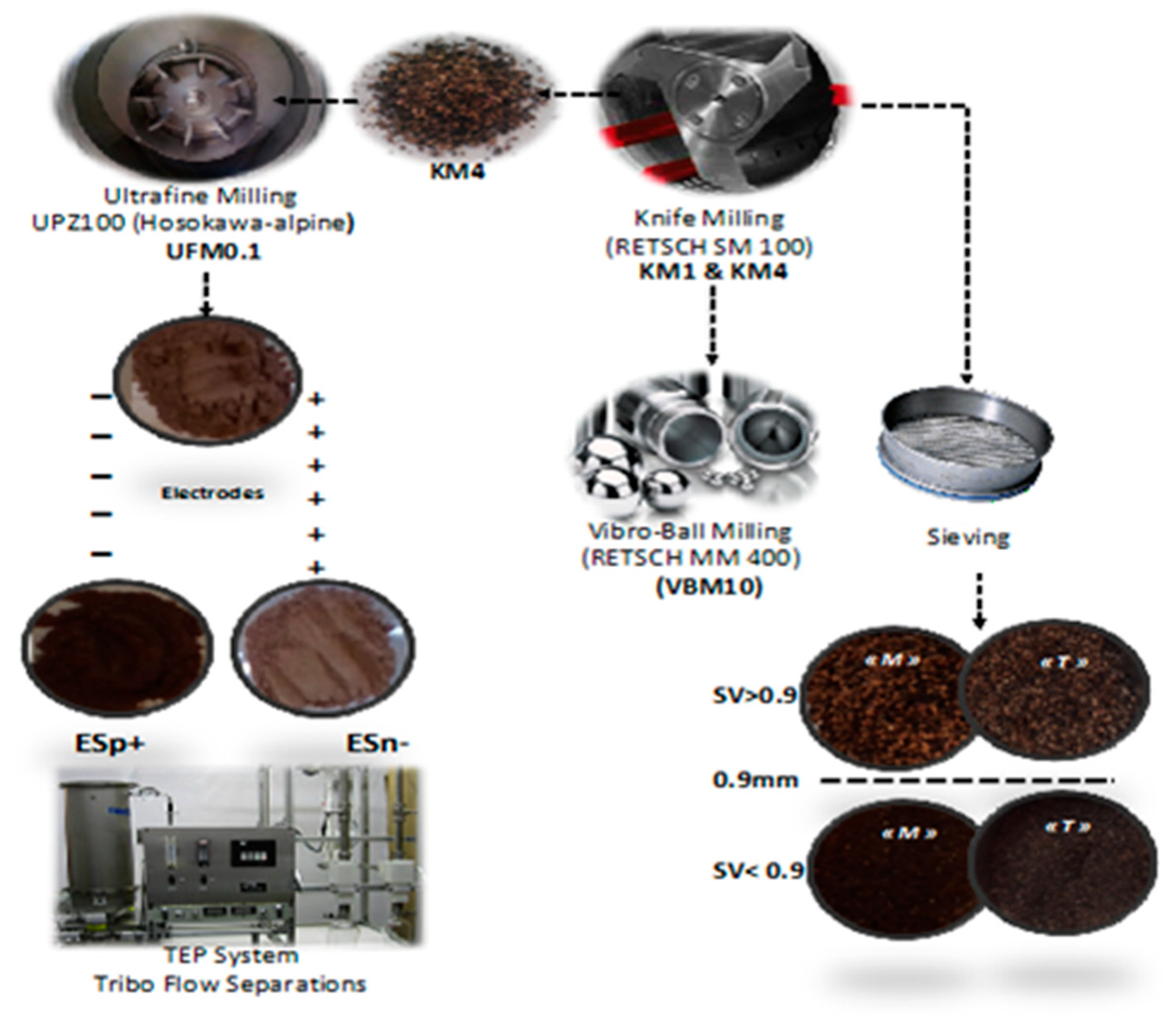
3.2. Mechanical Fractionation and Separation
3.3. Biochemical Methane Potential (BMP) Tests
3.4. Analysis
3.5. Specific Energy Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Federici, E.; Massaccesi, L.; Pezzolla, D.; Fidati, L.; Montalbani, E.; Proietti, P.; Nasini, L.; Regni, L.; Scargetta, S.; Gigliotti, G. Short-term modifications of soil microbial community structure and soluble organic matter chemical composition following amendment with different solid olive mill waste and their derived composts. Appl. Soil Ecol. 2017, 119, 234–241. [Google Scholar] [CrossRef]
- Miranda, T.; Arranz, J.I.; Montero, I.; Román, S.; Rojas, C.V.; Nogales, S. Characterization and combustion of olive pomace and forest residue pellets. Fuel Process. Technol. 2012, 103, 91–96. [Google Scholar] [CrossRef]
- Missaoui, A.; Bostyn, S.; Belandria, V.; Cagnon, B.; Sarh, B.; Gökalp, I. Hydrothermal carbonization of dried olive pomace: Energy potential and process performances. J. Anal. Appl. Pyrolysis 2017, 128, 281–290. [Google Scholar] [CrossRef]
- Benavente, V.; Fullana, A.; Berge, N.D. Life cycle analysis of hydrothermal carbonization of olive mill waste: Comparison with current management approaches. J. Clean. Prod. 2017, 142, 2637–2648. [Google Scholar] [CrossRef]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Fezzani, B.; Cheikh, R.B. Implementation of IWA anaerobic digestion model No. 1 (ADM1) for simulating the thermophilic anaerobic co-digestion of olive mill wastewater with olive mill solid waste in a semi-continuous tubular digester. Chem. Eng. J. 2008, 141, 75–88. [Google Scholar] [CrossRef]
- Almansa, A.R.; Rodriguez-Galan, M.; Borja, R.; Fermoso, F.G. Micronutrient dynamics after thermal pretreatment of olive mill solid waste. Bioresour. Technol. 2015, 191, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Rincón, B.; Bujalance, L.; Fermoso, F.G.; Martín, A.; Borja, R. Biochemical methane potential of two-phase olive mill solid waste: Influence of thermal pretreatment on the process kinetics. Bioresour. Technol. 2013, 140, 249–255. [Google Scholar] [CrossRef]
- Gianico, A.; Braguglia, C.M.; Mescia, D.; Mininni, G. Ultrasonic and thermal pretreatments to enhance the anaerobic bioconversion of olive husks. Bioresour. Technol. 2013, 147, 623–626. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Gidarakos, E. Chemical pretreatment of lignocellulosic agroindustrial waste for methane production. Waste Manag. 2018, 71, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; de Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Chuetor, S.; Monlau, F.; Solhy, A.; Rouau, X. Eco-friendly dry chemo-mechanical pretreatments of lignocellulosic biomass: Impact on energy and yield of the enzymatic hydrolysis. Appl. Energy 2014, 113, 97–105. [Google Scholar] [CrossRef]
- Hemery, Y.; Holopainen, U.; Lampi, A.-M.; Lehtinen, P.; Nurmi, T.; Piironen, V.; Edelmann, M.; Rouau, X. Potential of dry fractionation of wheat bran for the development of food ingredients, part II: Electrostatic separation of particles. J. Cereal Sci. 2011, 53, 9–18. [Google Scholar] [CrossRef]
- Basset, C.; Kedidi, S.; Barakat, A. Chemical- and solvent-free mechanophysical fractionation of biomass induced by tribo-electrostatic charging: Separation of proteins and lignin. ACS Sustain. Chem. Eng. 2016, 4, 4166–4173. [Google Scholar] [CrossRef]
- Hemery, Y.; Rouau, X.; Dragan, C.; Bilici, M.; Beleca, R.; Dascalescu, L. Electrostatic properties of wheat bran and its constitutive layers: Influence of particle size, composition, and moisture content. J. Food Eng. 2009, 93, 114–124. [Google Scholar] [CrossRef]
- Chuetor, S.; Luque, R.; Barron, C.; Solhy, A.; Rouau, X.; Barakat, A. Innovative combined dry fractionation technologies for rice straw valorization to biofuels. Green Chem. 2015, 17, 926–936. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Ortega-Martinez, E.; Ruiz-Filippi, G. Impact of milling, enzyme addition, and steam explosion on the solid waste biomethanation of an olive oil production plant. Bioprocess Biosyst. Eng. 2016, 39, 331–340. [Google Scholar] [CrossRef]
- Lammi, S.; Barakat, A.; Mayer-Laigle, C.; Djenane, D.; Gontard, N.; Angellier-Coussy, H. Dry fractionation of olive pomace as a sustainable process to produce fillers for biocomposites. Powder Technol. 2018, 326, 44–53. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Salvachúa, D.; Martínez, M.J.; Diaz, L.F.; Zamorano, M. Analysis of the relation between the cellulose, hemicellulose and lignin content and the thermal behavior of residual biomass from olive trees. Waste Manag. 2013, 33, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Wit, M.D.; Boom, R.M.; Schutyser, M.A.I. Lupine protein enrichment by milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2016, 33, 596–602. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer, C. Electrostatic separation as an entry into environmentally eco-friendly dry biorefining of plant materials. J. Chem. Eng. Process Technol. 2017, 8. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.E.; Edyvean, R.G.J. Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J. Hazard. Mater. 2008, 160, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.; Eastwick, C.; Kingman, S.; Giddings, D.; Lormor, S.; Lester, E. Overcoming the caking phenomenon in olive mill wastes. Ind. Crops Prod. 2017, 101, 92–102. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Santori, S.; Pomi, R.; Polettini, A.; Gidarakos, E. Effect of alkaline pretreatment on anaerobic digestion of olive mill solid waste. Waste Manag. 2016, 58, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rincón, B.; Bujalance, L.; Fermoso, F.G.; Martín, A.; Borja, R. Effect of ultrasonic pretreatment on biomethane potential of two-phase olive mill solid waste: Kinetic approach and process performance. Sci. World J. 2014, 2014, 648624. [Google Scholar] [CrossRef] [PubMed]
- Maamir, W. Effect of Fenton pretreatment on anaerobic digestion of olive mill wastewater and olive mill solid waste in mesophilic conditions. Int. J. Green Energy 2017, 5075, 555–560. [Google Scholar] [CrossRef]
- Carrere, H.; Rafrafi, Y.; Battimelli, A.; Torrijos, M.; Delgenes, J.P.; Motte, C. Improving methane production during the codigestion of waste-activated sludge and fatty wastewater: Impact of thermo-alkaline pretreatment on batch and semi-continuous processes. Chem. Eng. J. 2012, 210, 404–409. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H.; Zhang, H. Ball milling pretreatment of corn stover for enhancing the efficiency of enzymatic hydrolysis. Appl. Biochem. Biotech. 2010, 162, 1872–1880. [Google Scholar] [CrossRef]
- Martínez, L.; Ben, S.; Alami, D.; Hodaifa, G.; Faur, C.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Adsorption of iron on crude olive stones. Ind. Crops Prod. 2010, 32, 467–471. [Google Scholar] [CrossRef]
- Bartocci, P.; Amico, M.D.; Moriconi, N.; Bidini, G.; Fantozzi, F. Pyrolysis of olive stone for energy purposes. Energy Procedia 2015, 82, 374–380. [Google Scholar] [CrossRef]
- Hammoui, Y.; Molina-boisseau, S.; Duval, A.; Djerrada, N.; Adjeroud, N.; Remini, H.; Dahmoune, F.; Madani, K. Preparation of plasticized wheat gluten/olive pomace powder biocomposite: Effect of powder content and chemical modi fi cations. Mater. Des. 2015, 87, 742–749. [Google Scholar] [CrossRef]
- Monlau, F.; Latrille, E.; Carvalho, A.; Costa, D.; Steyer, J.; Carrère, H. Enhancement of methane production from sunflower oil cakes by dilute acid pretreatment. Appl. Energy 2013, 102, 1105–1113. [Google Scholar] [CrossRef]
- Sambusiti, C.; Ficara, E.; Malpei, F.; Steyer, J.P.; Carrère, H. Influence of alkaline pre-treatment conditions on structural features and methane production from ensiled sorghum forage. Chem. Eng. J. 2012, 211–212, 488–492. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Monlau, F.; Trably, E.; Barakat, A.; Hamelin, J.; Steyer, J.-P.; Carrere, H. Two-stage alkaline-enzymatic pretreatments to enhance biohydrogen production from sunflower stalks. Environ. Sci. Technol. 2013, 47, 12591–12599. [Google Scholar] [CrossRef]
- Mcmahon, A.; Lu, H.; Butovich, I.A. The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids 2013, 48, 513–525. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Bossel, U. Does a Hydrogen Economy Make Sense? In Proceedings of the IEEE International Symposium on Performance Analysis of Systems and Software, Austin, TX, USA, 19–21 March 2006; pp. 1826–1837. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Sample | M | T | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VBM10 | SV < 0.9 | SV > 0.9 | VBM10 | SV < 0.9 | SV > 0.9 | UFM0.1 | ESn− | ESp+ | |
| Yield (%) | 100 | 27 ± 1 | 73 ± 1 | 100 | 26 ± 2 | 74 ± 2 | 100 | 65 ± 5 | 35 ± 3 |
| TS (% Wb) | 94 ± 2 | 94 ± 2 | 92 ± 2 | 91 ± 0.2 | 92 ± 0.1 | 92 ± 0.5 | 91 ± 0.0 | 91 ± 0.0 | 91 ± 0.0 |
| VS (% Wb) | 82 ± 3 | 83 ± 2 | 81 ± 2 | 80 ± 2 | 87 ± 3 | 81 ± 2 | 82 ± 2 | 80 ± 1 | 87 ± 2 |
| D50 (µm) | 113 ± 5 | 507 ± 22 | - | 210 ± 4 | 601 ± 17 | - | 54 ± 2 | 49 ± 2 | 75 ± 3 |
| Elemental Analysis | |||||||||
| C (%) | 48 ± 0.1 | 51 ± 0.1 | 48 ± 0.1 | 48 ± 0.1 | 49 ± 0.1 | 48 ± 0.1 | 48 ± 1 | - | - |
| H (%) | 7 ± 0.1 | 7 ± 0.2 | 6 ± 0.3 | 7 ± 0.01 | 6 ± 0.1 | 6 ± 0.3 | 7 ± 0.01 | - | - |
| N (%) | 0.5 ± 0.02 | 1.5 ± 0.06 | 0.3 ± 0.09 | 0.6 ± 0.04 | 2 ± 0.004 | 0.5 ± 0.1 | 0.6 ± 0.04 | 0.5 ± 0.03 | 1.4 ± 0.1 |
| S (%) | 0.1 ± 0.002 | 0.2 ± 0.03 | 0.04 ± 0.002 | 0.05 ± 0.005 | 0.2 ± 0.02 | 0.1 ± 0.004 | 0.05 ± 0.005 | - | - |
| Hemicellulose(% Db) | 11 ± 0.2 | 4 ± 0.4 | 13 ± 0.3 | 16 ± 0.3 | 11 ± 1 | 17 ± 0.3 | 17 ± 1 | 20 ± 1 | 11 ± 0.4 |
| Cellulose(% Db) | 11 ± 0.1 | 5 ± 0.2 | 14 ± 1 | 10 ± 2 | 10 ± 1 | 11 ± 1 | 11 ± 1 | 15 ± 1 | 9 ± 0.4 |
| Lignin (% Db) | 37 ± 2 | 40 ± 1 | 30 ± 0.4 | 31 ± 0.03 | 34 ± 3 | 30 ± 4 | 36 ± 2 | 31 ± 2 | 41 ± 3 |
| Lipids (%Wb) | 14 ± 0.4 | 30 ± 0.8 | 9 ± 0.2 | 6 ± 0.2 | 6 ± 0.2 | 3 ± 0.1 | 6 ± 0.2 | - | - |
| Proteins (% Wb) | 3 ± 0.1 | 9 ± 0.4 | 2 ± 0.6 | 3 ± 0.2 | 11 ± 0.02 | 3 ± 0.6 | 3 ± 0.2 | 3.1 ± 0.2 | 9 ± 0.7 |
| TMY 1 (mL/gVS) | 249 | 386 | 213 | 168 | 203 | 161 | 191 | - | - |
| Colour | |||||||||
| a* | 8 ± 0.3 | 8 ± 0.3 | 10 ± 0.1 | 8 ± 0.6 | 7 ± 0.3 | 8 ± 0.1 | 5 ± 0.4 | 5 ± 0.3 | 6 ± 0.4 |
| b* | 9 ± 0.6 | 19 ± 1 | 18 ± 0.3 | 16 ± 1 | 12 ± 1 | 16 ± 1 | 13 ± 1 | 17 ± 1 | 12 ± 1 |
| L* | 33 ± 1 | 28 ± 1 | 34.5 ± 0.5 | 47 ± 4 | 37 ± 1 | 44 ± 1 | 53 ± 2 | 63 ± 2 | 43 ± 1 |
| ∆E* | - | 5 | 9 | - | 11 | 3 | - | 10 | 6 |
| Samples | sCOD (mg/gVS) | sCOD (mg/L) | sPolyphenols (mg GAE/gVS) | EMY 1 (mL CH4/gVS) | EMY/TMY 2 (%) | Enhancement in BMP (% KM4) | Enhancement in BMP (% KM1) | Enhancement in BMP (% UFM0.1) | |
|---|---|---|---|---|---|---|---|---|---|
| M | KM4 | 151 ± 4 | 604 ± 16 | 7.4 ± 0.1 | 188 ± 18 | 76 | - | ||
| KM1 | 159 ± 2 | 636 ± 8 | 7.5 ± 0.2 | 246 ± 10 | 98 | +30 | |||
| VBM10 | 263 ± 1 | 1052 ± 4 | 12.1 ± 0.2 | 224 ± 7 | 90 | +19 | −9 | ||
| SV < 0.9 | 245 ± 1 | 980 ± 4 | 10.6 ± 0.2 | 298 ± 7 | 77 | +58 | +21 | ||
| SV > 0.9 | 121 ± 1 | 484 ± 4 | 5.2 ± 0.2 | 105 ± 12 | 49 | −44 | −57 | ||
| T | KM4 | 115 ± 4 | 460 ± 16 | 4.2 ± 0.1 | 98 ± 14 | 58 | - | ||
| KM1 | 156 ± 3 | 624 ± 12 | 5.3 ± 0.3 | 110 ± 5 | 65 | +12 | |||
| VBM10 | 162 ± 3 | 648 ± 12 | 7.7 ± 0.2 | 108 ± 15 | 64 | +10 | −2 | ||
| UFM0.1 | 197 ± 4 | 788 ± 16 | 12.0 ± 0.2 | 168 ± 8 | 88 | +71 | +53 | ||
| SV < 0.9 | 205 ± 7 | 820 ± 28 | 7.5 ± 1.8 | 140 ± 4 | 69 | +43 | +27 | ||
| SV > 0.9 | 140 ± 3 | 560 ± 12 | 6.4 ± 1.2 | 98 ± 7 | 61 | 0 | −11 | ||
| Esn− | 182 ± 5 | 728 ± 20 | 7.7 ± 0.2 | 163 ± 10 | - | +66 | +48 | −3 | |
| Esp+ | 223 ± 5 | 892 ± 20 | 13.8 ± 0.4 | 177 ± 18 | - | +81 | +61 | +5 | |
| Pretreatments | KM4 | KM1 | VBM10 | UFM0.1 | Sieving (SV < 0.9) | ES | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | M | T | M | T | M | T | T | M | T | T Esp+ |
| Energy consumed by each process (kWh/kg OP) | 0.03 ± 0.01 | 0.27 ± 0.09 | 19.75 ± 3.17 | 1.87 ± 0.12 | 0.075 ± 0.00 | 1.56 ± 0.11 | ||||
| Total energy consumed (kWh/kg OP) | 0.03 | 0.30 | 19.78 | 1.90 | 0.11 | 3.46 | ||||
| Energy input (kJ/gVS init *) | 0.14 | 0.15 | 1.32 | 1.36 | 86.53 | 88.91 | 8.56 | 0.48 | 0.48 | 15.58 |
| CH4 (mL/gVS **) | 188 ± 18 | 98 ± 2 | 246 ± 10 | 110 ± 5 | 224 ± 7 | 99 ± 2 | 168 ± 8 | 298 ± 7 | 140 ± 4 | 177 ± 17 |
| Amount Recovered in the Fraction (%) | 27 | 26 | 35 | |||||||
| Energy output (kJ/gVS init) | 6.5 | 3.4 | 8.5 | 3.8 | 6.1 | 3.4 | 5.8 | 2.8 | 1.2 | 2.2 |
| Energy balance (kJ CH4/gVS init) | +6.36 | +3.30 | +7.2 | +2.4 | −80.4 | −85.5 | −2.8 | +2.32 | +0.72 | −13.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elalami, D.; Carrère, H.; Abdelouahdi, K.; Oukarroum, A.; Dhiba, D.; Arji, M.; Barakat, A. Combination of Dry Milling and Separation Processes with Anaerobic Digestion of Olive Mill Solid Waste: Methane Production and Energy Efficiency. Molecules 2018, 23, 3295. https://doi.org/10.3390/molecules23123295
Elalami D, Carrère H, Abdelouahdi K, Oukarroum A, Dhiba D, Arji M, Barakat A. Combination of Dry Milling and Separation Processes with Anaerobic Digestion of Olive Mill Solid Waste: Methane Production and Energy Efficiency. Molecules. 2018; 23(12):3295. https://doi.org/10.3390/molecules23123295
Chicago/Turabian StyleElalami, Doha, Hélène Carrère, Karima Abdelouahdi, Abdallah Oukarroum, Driss Dhiba, Mohamed Arji, and Abdellatif Barakat. 2018. "Combination of Dry Milling and Separation Processes with Anaerobic Digestion of Olive Mill Solid Waste: Methane Production and Energy Efficiency" Molecules 23, no. 12: 3295. https://doi.org/10.3390/molecules23123295





