A Comparative Study of Advanced Stationary Phases for Fast Liquid Chromatography Separation of Synthetic Food Colorants
Abstract
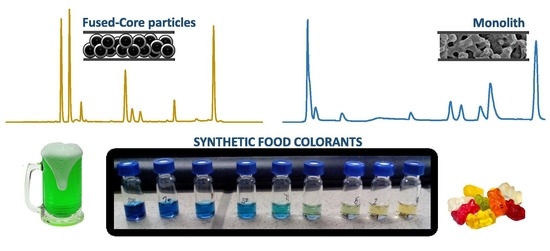
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation and Comparison
2.3. Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Chromatographic System
3.3. Preparation of Standard Solutions
3.4. Method I: Chromatographic Separation on Monolithic Column
3.5. Method II: Chromatographic Separation on Fused-Core Particle Column
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agroindustries to ensure expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Kus, E.; Eroglu, H.E. Genotoxic and cytotoxic effects of sunset yellow and brilliant blue, colorant food additives, on human blood lymphocytes. Pak. J. Pharm. Sci. 2015, 28, 227–230. [Google Scholar] [PubMed]
- Sarıkaya, R.; Selvi, M.; Erkoc, F. Evaluation of potential genotoxicity of five food dyes using the somatic mutation and recombination test. Chemosphere 2012, 88, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Axon, A.; May, F.E.B.; Gaughan, L.E.; Williams, F.M.; Blain, P.G.; Wright, M.C. Tartrazine and sunset yellow are xenoestrogens in a new screening assay to identify modulators of human oestrogen receptor transcriptional activity. Toxicology 2012, 298, 40–51. [Google Scholar] [CrossRef]
- Regulation EC No 1333/2008 on food additives. Off. J. Eur. Union 2008, L354, 16–33. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0016:0033:en:PDF (accessed on 24 October 2018).
- Leleu, C.; Boulitrop, C.; Bel, B.; Jeudy, G.; Vabres, P.; Collet, E. Quinoline Yellow dye-induced fixed food-and-drug eruption. Contact Dermat. 2013, 68, 187–188. [Google Scholar] [CrossRef]
- Stevens, L.J.; Kuczek, T.; Burgess, J.R.; Stochelski, M.A.; Arnold, L.E.; Galland, L. Mechanisms of behavioral, atopic and other reactions to artificial food colors in children. Nutr. Rev. 2013, 71, 268–281. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, C. Immune reactivity to food coloring. Altern. Ther. Health Med. 2015, 21, 52–62. [Google Scholar]
- Sierra-Rosales, P.; Toledo-Neira, C.; Squella, J.A. Electrochemical determination of food colorants in soft drinks using MWCNT-modified GCEs. Sens. Actuator B-Chem. 2017, 240, 1257–1264. [Google Scholar] [CrossRef]
- Yi, J.; Zeng, L.; Wu, Q.; Yang, L.; Xie, T. Sensitive simultaneous determination of synthetic food colorants in preserved fruit samples by capillary electrophoresis with contactless conductivity detection. Food Anal. Method 2017. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Pizzariello, A.; Susmel, S.; Perennes, F.; Bontempelli, G. A capillary electrophoresis microsystem for the rapid in-channel amperometric detection of synthetic dyes in food. J. Electroanal. Chem. 2007, 601, 1–7. [Google Scholar] [CrossRef]
- Heidarizadi, E.; Tabaraki, R. Simultaneous spectrophotometric determination of synthetic dyes in food samples after cloud point extraction using multiple response optimizations. Talanta 2016, 148, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, A.; Ghaedi, M.; Goudarzi, A. Optimization of ultrasound-assisted dispersive solid-phase microextraction based on nanoparticles followed by spectrophotometry for the simultaneous determination of dyes using experimental design. Ultrason. Sonochem. 2016, 32, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobańska, A.W.; Pyzowski, J.; Brzezińska, E. SPE/TLC/Densitometric quantification of selected synthetic food dyes in liquid foodstuffs and pharmaceutical preparations. J. Anal. Methods Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Wang, Y.; Wu, X.; Zhao, Y. Simultaneous Determination of Seven Synthetic Colorants in Wine by Dispersive Micro-Solid-Phase Extraction Coupled with Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2015, 53, 210–218. [Google Scholar] [CrossRef]
- de Araújo Sigueira Bento, W.; Lima, B.P.; Paim, A.P.S. Simultaneous determination of synthetic colorants in yogurt by HPLC. Food Chem. 2015, 183, 154–160. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. Application of high-performance liquid chromatography with diode array detector for simultaneous determination of 11 synthetic dyes in selected beverages and foodstuffs. Food Anal. Method 2017, 10, 3572–3588. [Google Scholar] [CrossRef]
- Lotfi, Z.; Mousavi, H.Z.; Sajjadi, S.M. A hyperbranched polyamidoamine dedrimer grafted onto magnetized graphene oxide as a sorbent for the extraction of synthetic dyes from foodstuff. Microchim. Acta 2017, 184, 4503–4512. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Feng, J.; Zeng, D.; Ding, L.; Liu, X.; Li, B. Research on the determination of 10 industrial dyes in foodstuffs. J. Chromatogr. Sci. 2017, 55, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Jian, N.; Qian, L.; Cao, W.; Xu, Q.; Li, J. Development and optimization of a novel sample preparation method cored on functionalized nanofibers mat-solid-phase extraction for the simultaneous efficient extraction of illegal anionic and cationic dyes in food. Anal. Bioanal. Chem. 2017, 409, 5697–5709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, J.; Jia, H.; Zhang, H. Liquid-liquid microextraction of synthetic pigments in beverages using a hydrophobic deep eutectic solvent. Food Chem. 2018, 243, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Davletbaeva, P.; Chocholouš, P.; Bulatov, A.; Šatínský, D.; Solich, P. Sub-1 min separation in sequential injection chromatography for determination of synthetic water-soluble dyes in pharmaceutical formulation. J. Pharm. Biomed. Anal. 2017, 143, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Amelin, V.G.; Korotkov, A.I.; Andoralov, A.M. Simultaneous determination of dyes of different classes in aquaculture products and spices using HPLC-high-resolution quadrupole time-of-flight mass spectrometry. J. Anal. Chem. 2017, 72, 183–190. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Miniaturized matrix solid-phase dispersion followed by liquid chromatography-tandem mass spectrometry for the quantification of synthetic dyes in cosmetics and foodstuffs used or consumed by children. J. Chromatogr. A 2017, 1529, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Núñez, O.; Gallart-Ayala, H.; Martins, C.P.B.; Lucci, P. New trends in fast liquid chromatography for food and environmental analysis. J. Chromatogr. A 2012, 1228, 298–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, N.; McCalley, D.V. Core-shell, ultrasmall particles, monoliths, and other support materials in high-performance liquid chromatography. Anal. Chem. 2016, 88, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Yasar, A. A Core-Shell Column Approach to Fast Determination of Synthetic Dyes in Foodstuffs by High-Performance Liquid Chromatography. Food Anal. Method 2018, 11, 1581–1590. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Quinoline yellow (95%), Tartrazine (99%), Sunset yellow (95%), Fast green FCF (98%), Green S (98%), Indigo carmine (98%) and Patent blue (97%) obtained from Fluka and Brilliant blue FCF (Sigma-Aldrich) are available from the authors. |




| Analyte | λ (nm) | tR (min) a | Repeatability | Peak Symmetry | wh b | Pc c | |
|---|---|---|---|---|---|---|---|
| tR, RSD (%) d | Peak Area, RSD (%) e | ||||||
| Monolithic Column | |||||||
| Tartrazine | 420 | 0.95 | 0.3 | 0.4; 0.1; 0.9 | 2.47 | 0.12 | 22.37 |
| Indigo carmine | 625 | 1.18 | 0.3 | 1.9; 0.4; 1.1 | 1.38 | 0.12 | 21.83 |
| Sunset yellow | 482 | 1.99 | 0.2 | 0.4; 0.1; 0.8 | 1.32 | 0.12 | 21.49 |
| Fast green | 625 | 6.53 | 0.1 | 0.3; 0.6; 0.7 | 1.19 | 0.12 | 22.37 |
| Brilliant blue | 625 | 6.91 | 0.1 | 0.3; 0.4; 0.5 | 1.09 | 0.12 | 21.66 |
| Green S | 625 | 7.67 | 0.1 | 1.6; 0.9; 1.0 | 1.53 | 0.16 | 16.72 |
| Patent blue | 625 | 7.96 | 0.1 | 0.7; 2.1; 0.6 | 2.32 | 0.18 | 15.12 |
| Quinoline yellow | 420 | 9.44 | 0.1 | 1.3; 0.4; 4.5 | 1.29 | 0.11 | 22.93 |
| Fused-core Column | |||||||
| Tartrazine | 420 | 1.51 | 0.2 | 1.5; 1.8; 1.0 | 1.60 | 0.02 | 60.38 |
| Indigo carmine | 625 | 1.74 | 0.2 | 1.7; 1.8; 1.5 | 1.82 | 0.03 | 48.50 |
| Sunset yellow | 482 | 2.05 | 0.1 | 1.6; 1.8; 1.3 | 1.77 | 0.03 | 50.14 |
| Green S | 625 | 3.26 | 0.1 | 3.7; 1.8; 1.1 | 1.33 | 0.05 | 29.50 |
| Fast green | 625 | 3.45 | 0.1 | 1.9; 2.0; 1.5 | 1.53 | 0.04 | 34.14 |
| Brilliant blue | 625 | 3.67 | 0.1 | 1.8; 1.8; 1.2 | 1.45 | 0.05 | 31.98 |
| Patent blue | 625 | 4.60 | 0.1 | 2.1; 2.0; 1.2 | 1.71 | 0.04 | 41.71 |
| Quinoline yellow | 420 | 5.67 | 0.1 | 1.8; 1.5; 0.9 | 1.38 | 0.04 | 39.51 |
| Analyte | Linear Range (mg L−1) a | Regression Equation | R2 | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|
| Monolith | |||||
| Tartrazine | 0.5–200 | y = 11,250x + 21,652 | 0.9973 | 0.03 | 0.09 |
| Quinoline yellow | 0.5–200 | y = 20,143x + 106,689 | 0.9934 | 0.03 | 0.11 |
| Sunset yellow | 2–200 | y = 2149x + 7683 | 0.9967 | 0.26 | 0.86 |
| Indigo carmine | 0.5–200 | y = 6834x + 23,541 | 0.9945 | 0.05 | 0.17 |
| Fast green | 0.5–200 | y = 28,509x + 96,884 | 0.9958 | 0.02 | 0.08 |
| Brilliant blue | 0.5–200 | y = 31,272x + 116,370 | 0.9954 | 0.02 | 0.06 |
| Green S | 0.5–200 | y = 23,582x + 89,043 | 0.9961 | 0.03 | 0.10 |
| Patent blue | 0.5–200 | y = 39,910x − 16,514 | 0.9997 | 0.02 | 0.06 |
| Fused-Core | |||||
| Tartrazine | 0.2–100 | y = 12,770x − 3835 | 0.9999 | 0.05 | 0.17 |
| Quinoline yellow | 0.2–100 | y = 30,986x − 13,046 | 0.9999 | 0.05 | 0.17 |
| Sunset yellow | 0.5–100 | y = 2042x − 42 | 0.9999 | 0.20 | 0.67 |
| Indigo carmine | 0.5–100 | y = 11,438x − 6214 | 0.9999 | 0.08 | 0.25 |
| Green S | 0.2–100 | y = 45,731x − 78,955 | 0.9955 | 0.02 | 0.05 |
| Fast green | 0.2–100 | y = 34,452x − 23,308 | 0.9998 | 0.02 | 0.06 |
| Brilliant blue | 0.2–100 | y = 33,215x − 22,166 | 0.9998 | 0.02 | 0.07 |
| Patent blue | 0.2–100 | y = 47,507x − 145,350 | 0.9908 | 0.01 | 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lhotská, I.; Solich, P.; Šatínský, D. A Comparative Study of Advanced Stationary Phases for Fast Liquid Chromatography Separation of Synthetic Food Colorants. Molecules 2018, 23, 3335. https://doi.org/10.3390/molecules23123335
Lhotská I, Solich P, Šatínský D. A Comparative Study of Advanced Stationary Phases for Fast Liquid Chromatography Separation of Synthetic Food Colorants. Molecules. 2018; 23(12):3335. https://doi.org/10.3390/molecules23123335
Chicago/Turabian StyleLhotská, Ivona, Petr Solich, and Dalibor Šatínský. 2018. "A Comparative Study of Advanced Stationary Phases for Fast Liquid Chromatography Separation of Synthetic Food Colorants" Molecules 23, no. 12: 3335. https://doi.org/10.3390/molecules23123335