Fluorine-Containing Dibenzoanthracene and Benzoperylene-Type Polycyclic Aromatic Hydrocarbons: Synthesis, Structure, and Basic Chemical Properties
Abstract
:1. Introduction
2. Results
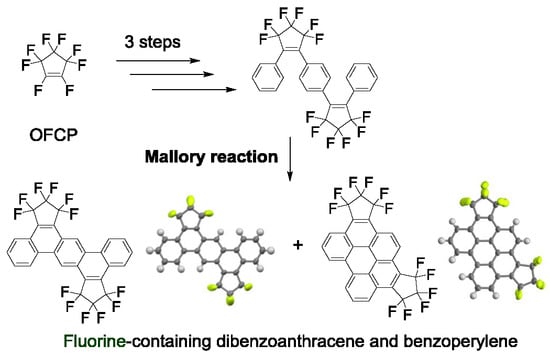
2.1. Synthesis of Fluorine-Containing Dibenzoanthracene (4a) and Benzoperylene (4b)
2.2. Molecular Structures of 3, 4a, and 4b
2.3. UV-Vis and Photoluminescence Spectra of 3, 4a, and 4b
2.4. Computational Study
3. Materials and Methods
3.1. General
3.2. Synthesis of 1-(4-bromophenyl)-2-phenyl-3,3,4,4,5,5-hexafluorocyclopentene (2)
3.3. Synthesis of 1,1′-[1,4-phenylenebis(3,3,4,4,5,5-hexafluorocyclopent-1-ene-2,1-diyl)]dibenzene (3)
3.4. Synthesis of 1,1,2,2,3,3,9,9,10,10,11,11-dodecafluoro-1,2,3,9,10,11-hexahydrobenzo[k]dicyclopenta[f,m]tetraphene (4a) and 1,1,2,2,3,3,10,10,11,11,12,12-dodecafluoro-1,2,3,10,11,12-hexahydrobenzo[pqr]dicyclopenta[b,n]perylene (4b)
3.5. X-Ray Crystallographic Analysis of 3, 4a, and 4b
3.6. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Kramer, J.H. Perfluoroalkylated PAH n-type semiconductors: Theory and experiment. In New Fluorinated Carbons: Fundamentals and Applications; Boltalina, O.V., Nakajima, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 155–176. [Google Scholar]
- Sakamoto, Y.; Suzuki, T.; Kobayashi, M.; Gao, Y.; Fukai, Y.; Inoue, Y.; Sato, F.; Tokito, S. Perfluoropentacene: High-Performance p−n junctions and complementary circuits with pentacene. J. Am. Chem. Soc. 2004, 126, 8138–8140. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, E.D.; Selig, H.; Lin, C.H.; Rabinovitz, M.; Agranat, I. Reaction of xenon difluoride with polycyclic aromatic hydrocarbons. fluorination of pyrene. J. Org. Chem. 1975, 40, 3793–3794. [Google Scholar] [CrossRef]
- Laali, K.K.; Tanaka, M.; Forohar, F.; Cheng, M.; Fetzer, J.C. Facile one-pot fluorination of polycyclic aromatic hydrocarbons (PAHs) with N-fluoro-2,4-dinitroimidazole; scope and limitation. J. Fluorine Chem. 1998, 91, 185–190. [Google Scholar] [CrossRef]
- Lee, H.G.; Milner, P.J.; Buchwald, S.L. Pd-catalyzed nucleophilic fluorination of aryl bromides. J. Am. Chem. Soc. 2014, 136, 3792–3795. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Ishida, N.; Ando, K.; Hashimoto, Y.; Shigaki, A.; Kikushima, K.; Ogoshi, S. Cu(I)-catalyzed pentafluoroethylation of aryl iodides in the presence of tetrafluoroethylene and cesium fluoride: Determining the route to key pentafluoroethyl Cu(I) intermediate. Chem. Eur. J. 2018, 24, 9794–9798. [Google Scholar] [CrossRef] [PubMed]
- Fuchibe, K.; Morikawa, T.; Ueda, R.; Okauchi, T.; Ichikawa, J. Pinpoint-fluorinated phenanthrene synthesis based on C–F bond activation of difluoroalkenes. J. Fluorine Chem. 2015, 179, 106–115. [Google Scholar] [CrossRef]
- Fuchibe, K.; Shigeno, K.; Zhao, N.; Aihara, H.; Akisaka, R.; Morikawa, T.; Fujita, T.; Yamakawa, K.; Shimada, T.; Ichikawa, J. Pinpoint-fluorinated polycyclic aromatic hydrocarbons (F-PAHs): Syntheses of difluorinated subfamily and their properties. J. Fluorine Chem. 2017, 203, 173–184. [Google Scholar] [CrossRef]
- Fuchibe, K.; Morikawa, T.; Shigeno, K.; Fujita, T.; Ichikawa, J. Pinpoint-fluorinated phenacenes: New synthesis and solubility enhancement strategies. Org. Lett. 2015, 17, 1126–1129. [Google Scholar] [CrossRef]
- Kikuzawa, Y.; Mori, T.; Takeuchi, H. Synthesis of 2,5,8,11,14,17-Hexafluoro-hexa-peri-hexabenzocoronene for n-Type organic field-effect transistors. Org. Lett. 2007, 9, 4817–4820. [Google Scholar] [CrossRef]
- Mallory, F.B.; Wood, C.S. Photochemistry of stilbenes. IV. The preparation of substituted phenanthrenes. J. Org. Chem. 1964, 29, 3373–3377. [Google Scholar]
- Mallory, F.B.; Wood, C.S.; Gordon, J.T. Photochemistry of stilbenes. III. Some aspects of the mechanism of photocyclization to phenanthrenes. J. Am. Chem. Soc. 1964, 86, 3094–3102. [Google Scholar] [CrossRef]
- Jørgensen, K.B. Photochemical oxidative cyclisation of stilbenes and stilbenoids—The Mallory reaction. Molecules 2010, 15, 4334–4358. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Sinha, S.; Pradhan, P.; Caruso, A.; Liebowitz, D.; Parrish, D.; Rossi, M.; Zajc, B. Regiospecifically fluorinated polycyclic aromatic hydrocarbons via julia−kocienski olefination and oxidative photocyclization. Effect of fluorine atom substitution on molecular shape. J. Org. Chem. 2016, 81, 3983–3993. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Mah, H.; Chaturvedi, S.; Jeknic, T.M.; Baird, W.M.; Katz, A.K.; Carrell, H.L.; Glusker, J.P.; Okazaki, T.; Laali, K.K.; et al. Synthetic, crystallographic, computational, and biological studies of 1,4-difluorobenzo[c]phenanthrene and its metabolites. J. Org. Chem. 2007, 72, 7625–7633. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Ando, M.; Shiota, T.; Izumiya, H.; Kubota, T. Efficient synthesis of fluorinated phenanthrene monomers using Mallory reaction and their copolymerization. Macromolecules 2017, 50, 865–871. [Google Scholar] [CrossRef]
- Yamada, S.; Konno, T.; Ishihara, T.; Yamanaka, H. Reaction of octafluorocyclopentene with various carbon nucleophiles. J. Fluorine Chem. 2005, 126, 125–133. [Google Scholar] [CrossRef]
- Ito, N.; Hirose, T.; Matsuda, K. Facile photochemical synthesis of 5,10-disubstituted [5]Helicenes by removing molecular orbital degeneracy. Org. Lett. 2014, 16, 2502–2505. [Google Scholar] [CrossRef]
- Blum, J.; Zimmerman, M. Photocyclization of substituted 1,4-distyrylbenzenes to dibenz[a,h] anthracenes. Tetrahedron 1972, 28, 275–280. [Google Scholar] [CrossRef]
- Hiroto, S.; Suzuki, K.; Kamiya, H.; Shinokubo, H. Synthetic protocol for diarylethenes through Suzuki–Miyaura coupling. Chem. Commun. 2011, 47, 7149–7151. [Google Scholar] [CrossRef]
- Baker, R.J.; Colavita, P.E.; Murphy, D.M.; Platts, J.A.; Wallis, J.D. Fluorine-fluorine interactions in the solid state: An experimental and theoretical study. J. Phys. Chem. A 2012, 116, 1435–1444. [Google Scholar] [CrossRef]
- Munakata, M.; Wu, L.P.; Ning, G.L.; Kuroda-Sowa, T.; Maekawa, M.; Suenaga, Y.; Maeno, N. Construction of metal sandwich systems derived from assembly of silver(I) complexes with polycyclic aromatic compounds. J. Am. Chem. Soc. 1999, 121, 4968–4976. [Google Scholar] [CrossRef]
- Hirayama, S.; Sakai, H.; Araki, Y.; Tanaka, M.; Imakawa, M.; Wada, T.; Takenobu, T.; Hasobe, T. Systematic control of the excited-state dynamics and carrier-transport properties of functionalized benzo[ghi]perylene and coronene derivatives. Chem. Eur. J. 2014, 20, 9081–9093. [Google Scholar] [CrossRef] [PubMed]
- Umeda, R.; Miyake, S.; Nishiyama, Y. Synthesis of dibenz[a,h]anthracenes by Pd-catalyzed intramolecular double-cyclization of (Z,Z)-p-styrylstilbenes. Chem. Lett. 2012, 41, 215–217. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro. 2015. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 11 December 2018).
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Sample Availability: Not available. |




| F-PAH | UV-Vis a λmax, nm (ε, M−1 cm−1) | Photoluminescence λem, nm |
|---|---|---|
| 4a | 292 (47000), 301 (64000), 322 (10400), 336 (10000), 352 (11000), 380 (1120), 401 (750) | 411, 433 |
| 4b | 284 (13400), 298 (14600), 309 (17600), 379 (6600), 401 (7100) | 418, 440 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotsu, O.; Shiota, T.; Fukumoto, H.; Kawasaki-Takasuka, T.; Yamazaki, T.; Yajima, T.; Agou, T.; Kubota, T. Fluorine-Containing Dibenzoanthracene and Benzoperylene-Type Polycyclic Aromatic Hydrocarbons: Synthesis, Structure, and Basic Chemical Properties. Molecules 2018, 23, 3337. https://doi.org/10.3390/molecules23123337
Gotsu O, Shiota T, Fukumoto H, Kawasaki-Takasuka T, Yamazaki T, Yajima T, Agou T, Kubota T. Fluorine-Containing Dibenzoanthracene and Benzoperylene-Type Polycyclic Aromatic Hydrocarbons: Synthesis, Structure, and Basic Chemical Properties. Molecules. 2018; 23(12):3337. https://doi.org/10.3390/molecules23123337
Chicago/Turabian StyleGotsu, Otohiro, Tomomi Shiota, Hiroki Fukumoto, Tomoko Kawasaki-Takasuka, Takashi Yamazaki, Tomoko Yajima, Tomohiro Agou, and Toshio Kubota. 2018. "Fluorine-Containing Dibenzoanthracene and Benzoperylene-Type Polycyclic Aromatic Hydrocarbons: Synthesis, Structure, and Basic Chemical Properties" Molecules 23, no. 12: 3337. https://doi.org/10.3390/molecules23123337