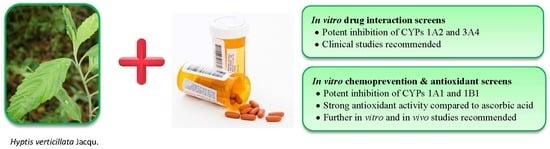
Inhibition of Cytochrome P450 Activities by Extracts of Hyptis verticillata Jacq.: Assessment for Potential HERB-Drug Interactions
Abstract
:1. Introduction
2. Results
2.1. Inhibition of CYP Activity by Aqueous Extract of Dried Aerial Plant Material
2.2. Inhibition of CYP1A2 Activity by Different Crude Extracts of H. verticillata (Aerial Plant)
2.3. Antioxidant Assays
2.4. Standardization of Dried Aerial Plant Aqueous Extract by RP-HPLC and LC-MS
2.4.1. RP-HPLC
2.4.2. LC-MS
2.5. Inhibition of CYP1A2 Activity by Key Phytochemicals Present in H. verticillata
2.5.1. Key Phytochemicals—Mixed Lignans, (-)-Yatein and β-Sitosterol
2.5.2. Key Phytochemicals—Oleanolic and Ursolic acid, Cadina-4,10(15)-Dien-3-One, Podophyllotoxin, 4′-Demethylpodophyllotoxin and Rosmarinic Acid
2.5.3. Inhibition of CYP1A2 Activity—Summary
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. Co-Expressed Human CYP Enzymes
4.3. Preparation of Crude Plant Extracts
4.3.1. Aqueous Extracts—Dried Plant (Leaf and Stem)
4.3.2. Aqueous Extracts—Fresh Plant (Leaf and Stem)
4.3.3. Ethanol Extracts—Dried and Fresh Plant (Leaf and Stem)
4.4. Standardization of Dried Plant Aqueous Extract (Leaf and Stem) by Reversed Phase HPLC (RP-HPLC) and LC-MS
4.4.1. High Performance Liquid Chromatography (HPLC)
4.4.2. Liquid Chromatography—Mass Spectrometry (LC-MS)
4.5. In Vitro Inhibition of CYP Activity
4.6. Controls Assessment
4.6.1. Positive Control Inhibition for CYP450 Assays (CYPs 1A1, 1A2, 1B1, 2D6, 3A4)
4.6.2. Solvent Impact on CYP1A2 Activity
4.6.3. Intrinsic Fluorescence
4.6.4. Metabolite Fluorescence Quenching
4.7. Antioxidant Assay
4.8. Data Analysis
4.8.1. Measuring Cytochrome P450 Inhibition
4.8.2. Antioxidant Assay
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A


Appendix B
| Phytochemicals Identified in H. verticillata (Published and Unpublished) | RP-HPLC Standard | Empirical Formula | m/z—Calculated Value 1 (Difference Between m/z Value and Mono-Isotopic Mass Where the Difference is ≤1.0) | |||||
|---|---|---|---|---|---|---|---|---|
| [M + H] + | [M + CH3] + | [M + H2O] + | [M + Na] + | [M + CH3OH] + | [M + K] + | |||
| Cadina-4,10(15)-dien-3-one | (−) | C15H22O | ||||||
| Cadina-10(15)-en-3-one | C15H22O | |||||||
| Aromadendr-1(10)-en-9-one | C15H22O | |||||||
| 7,11,15-Trimethyl-3-methylenehexadecane -1,2-diol | C20H40O2 | 330.3 (0.9) | ||||||
| Rosmarinic acid | C18H16O8 | 392.3 (0.97) | ||||||
| Deoxydehydropodophyllotoxin 2 | (+) | C22H18O7 | 409.1 (0.1) | 433.1 (0.7) | ||||
| Dehydrodesoxypodophyllotoxin | C22H18O7 | 409.1 (0.1) | 433.1 (0.7) | |||||
| ß-Apopicropodophyllin | C22H20O7 | 419.0 (0.4) | ||||||
| Hyptinin | C22H20O7 | 419.0 (0.4) | ||||||
| Isodeoxypodophyllotoxin | C22H22O7 | 437.1 (0.2) | ||||||
| Deoxypicropodophyllin | C22H22O7 | 437.1 (0.2) | ||||||
| 4-Demethylpodophyllotoxin | (+) | C21H20O8 | 418.1 (0.3) | 432.1 (0.3) | ||||
| (-)-Yatein | (+) | C21H20O8 | 418.2 (0.2) | 432.2 (0.2) | ||||
| Dehydropodophyllotoxin 2 | (+) | C22H18O8 | 433.0 (0.6) | |||||
| Podophyllotoxin | (+) | C22H22O8 | 432.1 (0.3) | 437.0 (0.3) | ||||
| ß-Peltatin | C22H22O8 | 432.1 (0.3) | 437.0 (0.3) | |||||
| ß -Sitosterol 3 | (−) | C29H50O | 432.4 (0.0) | 437.3 (0.0) | ||||
| Oleanolic acid 4 | (+) | C30H48O3 | ||||||
References
- WHO. WHO Traditional Medicine Strategy 2002–2005. Available online: http://whqlibdoc.who.int/ hq/2002/WHO_EDM_TRM_2002.1.pdf (14 February 2018).
- Cordell, G.A. Sustainable medicines and global health care. Planta Med. 2011, 77, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Picking, D.; Younger, N.; Mitchell, S.; Delgoda, R. The prevalence of herbal medicine home use and concomitant use with pharmaceutical medicines in Jamaica. J. Ethnopharmacol. 2011, 137, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Younger, N.; Aiken, W.; Brady-West, D.; Delgoda, R. Reliance on medicinal plant therapy among cancer patients in Jamaica. Cancer Causes Control 2017, 28, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Delgoda, R.; Ellington, C.; Barrett, S.; Gordon, N.; Clarke, N.; Younger, N. The practice of polypharmacy involving herbal and prescription medicines in the treatment of Diabetes mellitus, hypertension and gastrointestinal disorders in Jamaica. West. Indian Med. J. 2004, 53, 400–405. [Google Scholar] [PubMed]
- Delgoda, R.; Younger, N.; Barrett, C.; Braithwaite, J.; Davis, D. The prevalence of herbs use in conjunction with conventional medicines in Jamaica. Complement. Ther. Med. 2010, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Djuv, A.; Nilsen, O.G.; Steinsbekk, A. The co-use of conventional drugs and herbs among patients in Norwegian general practice: A cross-sectional study. BMC Complement. Altern. Med. 2013, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Delgoda, R.; Westlake, A.C. Herbal interactions involving cytochrome P450 enzymes: A mini review. Toxicol. Rev. 2004, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002, 72, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Shields, M. The Effect of Jamaican Medicinal Plants on the Activities of Cytochrome P450 Enzymes. Ph.D. Thesis, University of the West Indies, Kingston, Jamaica, 2006. [Google Scholar]
- Picking, D. The Contemporary Use of Medicinal Plants in Jamaica & Assessment of Potential Medicinal Plant-Drug Interactions of Select Plants. Ph.D. Thesis, University of the West Indies, Kingston, Jamaica, 2014. [Google Scholar]
- Delgoda, R.; Picking, D. Potential Drug Interactions for Commonly Used Medicinal Plants and Foods in Jamaica; Natural Products Institute, University of the West Indies: Kingston, Jamaica, 2015. [Google Scholar]
- Nwokocha, C.R.; Owu, D.U.; McLaren, M.; Murray, J.; Delgoda, R.; Thaxter, K.; McCalla, G.; Young, L. Possible mechanisms of action of the aqueous extract of Artocarpus altilis (breadfruit) leaves in producing hypotension in normotensive sprague-dawley rats. Pharm. Biol. 2012, 50, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Nwokocha, C.R.; Owu, D.U.; Kinlocke, K.; Murray, J.; Delgoda, R.; Thaxter, K.; McCalla, G.; Young, L. Possible mechanism of action of the hypotensive effect of Peperomia pellucida and interactions between human cytochrome P450 enzymes. Med. Aromat. Plants 2012, 1. [Google Scholar] [CrossRef]
- Murray, J.; Picking, D.; Lamm, A.; McKenzie, J.; Hartley, S.; Watson, C.; Williams, L.; Lowe, H.; Delgoda, R. Significant inhibitory impact of dibenzyl trisulfide and extracts of Petiveria alliacea on the activities of major drug-metabolizing enzymes in vitro: An assessment of the potential for medicinal plant-drug interactions. Fitoterapia 2016, 111, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.; Niazi, U.; Badal, S.; Yee, T.; Sutcliffe, M.J.; Delgoda, R. Inhibition of CYP1A1 by quassinoids found in Picrasma Excelsa. Planta Med. 2009, 75, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Picking, D.; Delgoda, R.; Younger, N.; Germosen-Robineau, L.; Boulogne, I.; Mitchell, S. Tramil ethnomedicinal survey in Jamaica. J. Ethnopharmacol. 2015, 169, 314–327. [Google Scholar] [CrossRef] [PubMed]
- TRAMIL. Program of Applied Research for Traditional Popular Medicine in the Caribbean. 2011. Available online: http://www.tramil.net/.
- Picking, D.; Delgoda, R.; Boulogne, I.; Mitchell, S. Hyptis verticillata jacq: A review of its traditional uses, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2013, 147, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Porter, R. Secondary Metabolites from Hyptis verticillata and Biotransformations of Steroids by Exophiala sp. Ph.D. Thesis, University of the West Indies, Mona, Jamaica, 1994. [Google Scholar]
- Biggs, D. Isolation and Characterisation of Phytochemicals from Three Folklore Medicinal Plants. Ph.D. Thesis, University of the West Indies, Kingston, Jamaica, 2008. [Google Scholar]
- Markowitz, J.S.; von Moltke, L.L.; Donovan, J.L. Predicting interactions between conventional medications and botanical products on the basis of in vitro investigations. Mol. Nutr. Food Res. 2008, 52, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A. Drug-Drug Interactions, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 78. [Google Scholar]
- Strandell, J.; Wahlin, S. Pharmacodynamic and pharmacokinetic drug interactions reported to Vigibase, the WHO global individual case safety report database. Eur. J. Clin. Pharmacol. 2011, 67, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Ponnusankar, S.; Pandit, S.; Hazam, P.K.; Ahmmed, M.; Mukherjee, K. Botanicals as medicinal food and their effects on drug metabolizing enzymes. Food Chem. Toxicol. 2011, 49, 3142–3153. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D. Medical Herbalism: The Science and Practice of Herbal Medicine; Healing Arts Press: Rochester, NY, USA, 2003. [Google Scholar]
- Venkataramanan, R.; Komoroski, B.; Strom, S. In vitro and in vivo assessment of herb drug interactions. Life Sci. 2006, 78, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Klimo, K.; Heiss, E.; Neumann, I.; Gamal-Eldeen, A.; Knauft, J.; Liu, G.Y.; Sitthimonchai, S.; Frank, N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003, 523–524, 163–172. [Google Scholar] [CrossRef]
- Oyama, T.; Kagawa, N.; Kunugita, N.; Kitagawa, K.; Ogawa, M.; Yamaguchi, T.; Suzuki, R.; Kinaga, T.; Yashima, Y.; Ozaki, S.; et al. Expression of cytochrome P450 in tumor tissues and its association with cancer development. Front. Biosci. 2004, 9, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.D.; Njar, V.C. Targeting cytochrome P450 enzymes: A new approach in anti-cancer drug development. Bioorg. Med. Chem. 2007, 15, 5047–5060. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Gomez, A.; Karlgren, M.; Sim, S.C.; Ingelman-Sundberg, M. Molecular genetics and epigenetics of the cytochrome P450 gene family and its relevance for cancer risk and treatment. Hum. Genet. 2010, 127, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tokizane, T.; Shiina, H.; Igawa, M.; Enokida, H.; Urakami, S.; Kawakami, T.; Ogishima, T.; Okino, S.T.; Li, L.C.; Tanaka, Y.; et al. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin. Cancer Res. 2005, 11, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Delgoda, R. A patent review on the development of human cytochrome P450 inhibitors. Expert Opin. Ther. Pat. 2014, 24, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.D.; Hibbert, S.; Porter, R.B.R.; Bailey-Shaw, Y.A.; Green, C.E. Jamaican plants with in vitro anti-oxidant activity. In Biologically Active Natural Products for the 21st Century; Williams, L.A.D., Reese, P.B., Eds.; Research Signpost: Trivandrum, India, 2006; p. 124. [Google Scholar]
- Wang, H.; Wang, Z.; Guo, W. Comparative determination of ursolic acid and oleanolic acid of Macrocarpium officinalis (Sieb. Et Zucc.) Nakai by RP-HPLC. Ind. Crops Prod. 2008, 28, 328–332. [Google Scholar] [CrossRef]
- Xu, X.-H.; Su, Q.; Zang, Z.-H. Simultaneous determination of oleanolic acid and ursolic acid by rp-hplc in the leaves of Eriobotrya japonica Lindl. J. Pharm. Anal. 2012, 2, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.; Park, H.; Kim, J.; Kim, C.; Shim, I.; Kim, N.; Han, S.; Lim, S. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004, 74, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Usia, T.; Watabe, T.; Kadota, S.; Tezuka, Y. Potent CYP3A4 inhibitory constituents of Piper cubeba. J. Nat. Prod. 2005, 68, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Julsing, M.K.; Vasilev, N.P.; Schneidman-Duhovny, D.; Muntendam, R.; Woerdenbag, H.J.; Quax, W.J.; Wolfson, H.J.; Ionkova, I.; Kayser, O. Metabolic stereoselectivity of cytochrome P450 3A4 towards deoxypodophyllotoxin: In silico predictions and experimental validation. Eur. J. Med. Chem. 2008, 43, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M. Ethnobotany and natural products: The search for new molecules, new treatments of old diseases or a better understanding of indigenous cultures? Curr. Top. Med. Chem. 2003, 3, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Sun, Z.L.; Liu, T.; Gibbons, S.; Zhang, W.J.; Qing, M. Flavonoids from sophora moorcroftiana and their synergistic antibacterial effects on mrsa. Phytother. Res. 2014, 28, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Tarkang, P.A.; Appiah-Opong, R.; Ofori, M.F.; Ayong, L.S.; Nyarko, A.K. Application of multi-target phytotherapeutic concept in malaria drug discovery: A systems biology approach in biomarker identification. Biomark. Res. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Peifer, M.; Weiss, J.; Sos, M.L.; Koker, M.; Heynck, S.; Netzer, C.; Fischer, S.; Rode, H.; Rauh, D.; Rahnenfuhrer, J.; et al. Analysis of compound synergy in high-throughput cellular screens by population-based lifetime modeling. PLoS ONE 2010, 5, e8919. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S. Network pharmacology for cancer drug discovery: Are we there yet? Future Med. Chem. 2012, 4, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Schweim, H.G. Synergistic Effects—Is it possible to make ‘the devil an angel’? Synergy 2017, 4, 8–12. [Google Scholar] [CrossRef]
- Pan, Y.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Pook, P.C.; Er, H.M.; Ong, C.E. In vitro effects of active constituents and extracts of Orthosiphon stamineus on the activities of three major human cdna-expressed cytochrome p450 enzymes. Chem. Biol. Interact. 2011, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Melillo de Magalhaes, P.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Usia, T.; Kadota, S.; Tezuka, Y. Constituents of Zingiber aromaticum and their CYP3A4 and CYP2D6 inhibitory activity. Chem. Pharm. Bull. 2005, 53, 333–335. [Google Scholar]
- Nair, V.D.; Foster, B.C.; Thor Arnason, J.; Mills, E.J.; Kanfer, I. In vitro evaluation of human cytochrome P450 and p-glycoprotein-mediated metabolism of some phytochemicals in extracts and formulations of african potato. Phytomedicine 2007, 14, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Willfor, S.M.; Smeds, A.I.; Holmbom, B.R. Chromatographic analysis of lignans. J. Chromatogr. A 2006, 1112, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.; Miller, V.; Penman, B. Microtiter plate assays for inhibition of human, drug metabolising cytochromes P450. Anal. Biochem. 1997, 248, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.; Wang, J.P.; Wang, Y.; Unadkat, J.D. Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab. Dispos. 1998, 26, 207–215. [Google Scholar] [PubMed]
- Busby, W.F., Jr.; Ackermann, J.M.; Crespi, C.L. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cdna-expressed human cytochromes P-450. Drug Metab. Dispos. 1999, 27, 246–249. [Google Scholar] [PubMed]
Sample Availability: Not available. |



| Phytochemicals Identified in H. verticillata by LC-MS | Empirical Formula | Mono-isotopic Mass (Da) 1 | Confirmation by RP-HPLC Standard |
|---|---|---|---|
| Cadina-10(15)-en-3-one | C15H22O | 218.167 | |
| Aromadendr-1(10)-en-9-one | C15H22O | 218.167 | |
| 7,11,15-Trimethyl-3-methylenehexadecane -1,2-diol | C20H40O2 | 312.303 | |
| Rosmarinic acid | C18H16O8 | 360.315 | |
| Deoxydehydropodophyllotoxin 2 | C22H18O7 | 394.105 | (+) |
| Dehydrodesoxypodophyllotoxin | C22H18O7 | 394.105 | |
| ß-Apopicropodophyllin | C22H20O7 | 396.121 | |
| Hyptinin | C22H20O7 | 396.121 | |
| Isodeoxypodophyllotoxin | C22H22O7 | 398.136 | |
| Deoxypicropodophyllin | C22H22O7 | 398.136 | |
| 4-Demethylpodophyllotoxin | C21H20O8 | 400.116 | (+) |
| (-)-Yatein | C21H20O8 | 400.152 | (+) |
| Dehydropodophyllotoxin 2 | C22H18O8 | 410.100 | (+) |
| Podophyllotoxin | C22H22O8 | 414.131 | (+) |
| ß-Peltatin | C22H22O8 | 414.131 | |
| Oleanolic acid 3 | C30H48O3 | 456.360 | (+) |
| Test Phytochemical | Concentration Range Tested [µg/mL] | Solvent (Highest % v/v) | IC50 (µg/mL) |
| H. verticillata aqueous extract (dried aerial material) | 0.25–60 | H2O | 1.9 ± 0.5 |
| Lignan Mix | 0.08–180 | DMF (2.0) | 61.8 ± 0.7 |
| -Dehydropodophyllotoxin -Deoxydehydropodophyllotoxin -4′-Demethyldesoxypodophyllotoxin -5′-Methoxydehydropodophyllotoxin -Dehydro-β-peltatin methyl ether | |||
| Test Phytochemical | Concentration Range Tested [µM] | Solvent (Highest % v/v) | IC50 (μM) |
| Podophyllotoxin | 2.9–369 | DMF (0.7) | No inhibition |
| 4′-Demethylpodophyllotoxin | 0.28–611 | DMF (0.7) | No inhibition |
| (-)-Yatein | 0.18–400 | DMF (1.2) | 71.9 ± 1.8 |
| Cadina-4,10(15)-dien-3-one | 0.28–623 | H2O | >500 |
| Oleanolic acid | 0.9–219 | DMF (1.3) | >100 |
| Ursolic acid | 0.05–117 | DMF (1.8) | >100 |
| β-Sitosterol | 5.1–410 | DMF (2.0) | 160 ± 3.4 |
| Furafylline—reference inhibitor | 0.04–96 | 1.4 ± 0.15 |
| Phytochemicals | Reported CYP Inhibition | IC50 Value (μM) | Reference |
|---|---|---|---|
| Podophyllotoxin | CYP3A4 | Potent inhibitor (IC50 not stated) | [40] |
| Dehydropodophyllotoxin | None | - | |
| Deoxydehydropodophyllotoxin | None | - | |
| 4′-Demethyldesoxypodophyllotoxin | None | - | |
| 4′-Demethylpodophyllotoxin | None | - | |
| 5′-Methoxydehydropodophyllotoxin | None | - | |
| Dehydro-β-peltatin methyl ether | None | - | |
| (-)-yatein | CYP3A4 CYP2D6 | 1.095.7 | [39] [39] |
| Oleanolic acid | CYP1A2 CYP2C8/C9/C19 CYP3A4 CYP2D6 | 143.5 >500 78.9 >500 | [38] [38] [38] [38] |
| Ursolic acid | CYP1A2 CYP2C8/C9/C19 CYP3A4 CYP2D6 | 352.4 >500 >500 438.9 | [38] [38] [38] [38] |
| Rosmarinic acid | CYP2C9 CYP2D6 CYP3A4 CYP3A4 | 318.9 >500 241.2 No impact | [49] [49] [49] [50] |
| ß-Sitosterol | CYP2D6 CYP3A4 CYP3A4/3A5 CYP2C19 | >100 >100 No impact No impact | [51] [51] [52] [52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picking, D.; Chambers, B.; Barker, J.; Shah, I.; Porter, R.; Naughton, D.P.; Delgoda, R. Inhibition of Cytochrome P450 Activities by Extracts of Hyptis verticillata Jacq.: Assessment for Potential HERB-Drug Interactions. Molecules 2018, 23, 430. https://doi.org/10.3390/molecules23020430
Picking D, Chambers B, Barker J, Shah I, Porter R, Naughton DP, Delgoda R. Inhibition of Cytochrome P450 Activities by Extracts of Hyptis verticillata Jacq.: Assessment for Potential HERB-Drug Interactions. Molecules. 2018; 23(2):430. https://doi.org/10.3390/molecules23020430
Chicago/Turabian StylePicking, David, Bentley Chambers, James Barker, Iltaf Shah, Roy Porter, Declan P Naughton, and Rupika Delgoda. 2018. "Inhibition of Cytochrome P450 Activities by Extracts of Hyptis verticillata Jacq.: Assessment for Potential HERB-Drug Interactions" Molecules 23, no. 2: 430. https://doi.org/10.3390/molecules23020430
APA StylePicking, D., Chambers, B., Barker, J., Shah, I., Porter, R., Naughton, D. P., & Delgoda, R. (2018). Inhibition of Cytochrome P450 Activities by Extracts of Hyptis verticillata Jacq.: Assessment for Potential HERB-Drug Interactions. Molecules, 23(2), 430. https://doi.org/10.3390/molecules23020430