Electronic Structure of C60/Zinc Phthalocyanine/V2O5 Interfaces Studied Using Photoemission Spectroscopy for Organic Photovoltaic Applications
Abstract
:1. Introduction
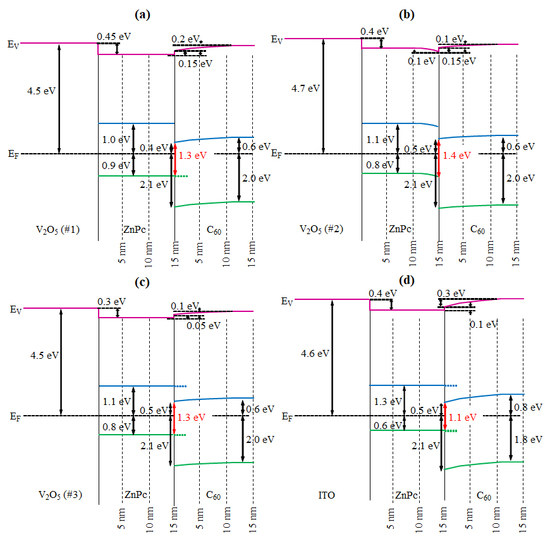
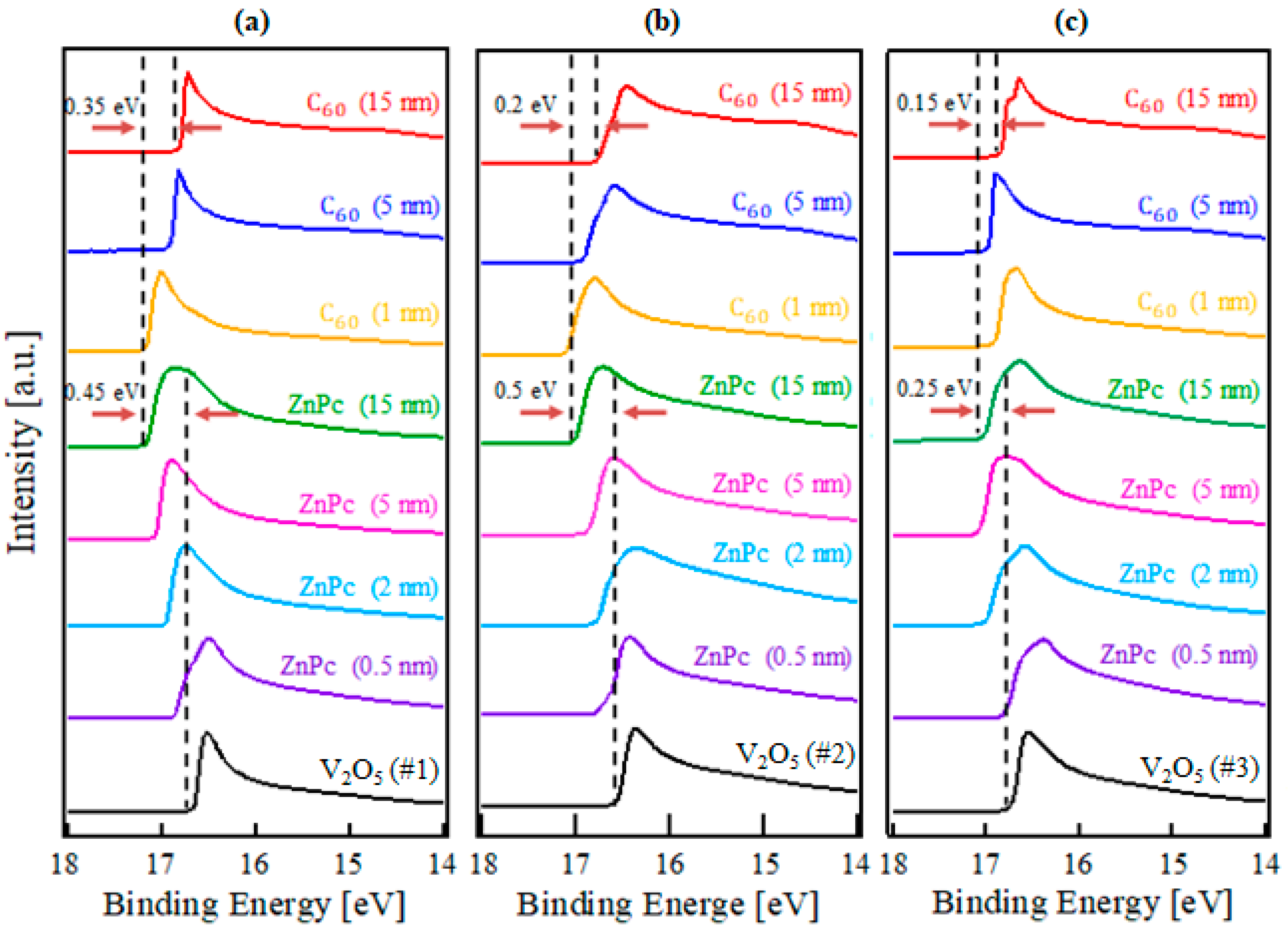
2. Results and Discussion
3. Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, Y.; Yaacobi-Gross, N.; Perumal, A.K.; Faber, H.A.; Vourlias, G.; Patsalas, P.A.; Bradley, D.D.C.; He, Z.Q.; Anthopoulos, T.D. Efficient organic solar cells using copper(I) iodide (CuI) hole transport layers. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Falke, S.M.; Rozzi, C.A.; Brida, D.; Maiuri, M.; Amato, M.; Sommer, E.; De Sio, A.; Rubio, A.; Cerullo, G.; Molinari, E.; et al. Coherent ultrafast charge transfer in an organic photovoltaic blend. Science 2014, 344, 1001–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, P.; Duraud, A.; Hancox, I.; Beaumont, N.; Mirri, G.; Tucker, J.H.R.; Hatton, R.A.; Shipman, M.; Jones, T.S. Halogenated boron subphthalocyanines as light harvesting electron acceptors in organic photovoltaics. Adv. Energy Mater. 2011, 1, 352–355. [Google Scholar] [CrossRef]
- Silvestri, F.; Marrocchi, A.; Seri, M.; Kim, C.; Marks, T.J.; Facchetti, A.; Taticchi, A. Solution-processable low-molecular weight extended arylacetylenes: Versatile p-Type semiconductors for field-effect transistors and bulk heterojunction solar cells. J. Am. Chem. Soc. 2010, 132, 6108–6123. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J. Organic photovoltaics: Technology and market. Sol. Energy Mater. Sol. C 2004, 83, 273–292. [Google Scholar] [CrossRef]
- Garcias-Morales, C.; Romero-Borja, D.; Maldonado, J.-L.; Roa, A.; Rodríguez, M.; García-Merinos, J.; Ariza-Castolo, A. Small molecules derived from Thieno[3,4-c]pyrrole-4,6-dione (TPD) and their use in solution processed organic solar cells. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, S.W.; Yi, Y. Interfacial electronic structure for high performance organic devices. Curr. Appl. Phys. 2016, 16, 1533–1549. [Google Scholar] [CrossRef]
- Kim, J.S.; Friend, R.H.; Grizzi, I.; Burroughes, J.H. Spin-cast thin semiconducting polymer interlayer for improving device efficiency of polymer light-emitting diodes. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Bhosle, V.; Prater, J.T.; Yang, F.; Burk, D.; Forrest, S.R.; Narayan, J. Gallium-doped zinc oxide films as transparent electrodes for organic solar cell applications. J. Appl. Phys. 2007, 102. [Google Scholar] [CrossRef]
- Murdoch, G.B.; Hinds, S.; Sargent, E.H.; Tsang, S.W.; Mordoukhovski, L.; Lu, Z.H. Aluminum doped zinc oxide for organic photovoltaics. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Zou, J.Y.; Li, C.Z.; Chang, C.Y.; Yip, H.L.; Jen, A.K.Y. Interfacial engineering of ultrathin metal film transparent electrode for flexible organic photovoltaic cells. Adv. Mater. 2014, 26, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.; Kim, Y.; Jung, Y.; Cheon, S.; Cho, S.; Cho, S.W.; Park, S.; Yi, Y.; Smith, K.E. Interfacial electronic structure of C-60/ZnPc/AZO on photoemission spectroscopy for organic photovoltaic applications. Chem. Phys. 2016, 478, 145–149. [Google Scholar] [CrossRef]
- Cattin, L.; Dahou, F.; Lare, Y.; Morsli, M.; Tricot, R.; Houari, S.; Mokrani, A.; Jondo, K.; Khelil, A.; Napo, K.; et al. MoO3 surface passivation of the transparent anode in organic solar cells using ultrathin films. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Beke, S. A review of the growth of V2O5 films from 1885 to 2010. Thin Solid Films 2011, 519, 1761–1771. [Google Scholar] [CrossRef]
- Morin, F.J. Oxides which show a metal-to-insulator transition at the neel temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Iida, Y.; Kaneko, Y.; Kanno, Y. Fabrication of pulsed-laser deposited V2O5 thin films for electrochromic devices. J. Mater. Process. Technol. 2008, 197, 261–267. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Z.; Xu, X.; Du, M.; Wang, T.; Jiang, Y. Impact of substrate temperature on the microstructure, electrical and optical properties of sputtered nanoparticle V2O5 thin films. Vacuum 2010, 85, 145–150. [Google Scholar] [CrossRef]
- Mousavi, M.; Kompany, A.; Shahtahmasebi, N.; Bagheri-Mohagheghi, M.M. Effect of S-doping on structural, optical and electrochemical properties of vanadium oxide thin films prepared by spray pyrolysis. Phys. Scr. 2013, 88. [Google Scholar] [CrossRef]
- Mousavi, M.; Kompany, A.; Shahtahmasebi, N.; Bagheri-Mohagheghi, M.M. Study of structural, electrical and optical properties of vanadium oxide condensed films deposited by spray pyrolysis technique. Adv. Manuf. 2013, 1, 320–328. [Google Scholar] [CrossRef]
- Piper, L.F.J.; Cho, S.W.; Zhang, Y.; DeMasi, A.; Smith, K.E.; Matsuura, A.Y.; McGuinness, C. Soft X-ray spectroscopy study of the element and orbital contributions to the electronic structure of copper hexadecafluoro-phthalocyanine. Phys. Rev. B 2010, 81, 1–12. [Google Scholar] [CrossRef]
- Chauhan, V.; Hatton, R.; Sullivan, P.; Jones, T.; Cho, S.W.; Piper, L.; deMasi, A.; Smith, K. Elucidating the factors that determine the open circuit voltage in discrete heterojunction organic photovoltaic cells. J. Mater. Chem. 2010, 20, 1173–1178. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454–456, 41–44. [Google Scholar] [CrossRef]
- Lee, S.T.; Hou, X.Y.; Mason, M.G.; Tang, C.W. Energy level alignment at Alq/metal interfaces. Appl. Phys. Lett. 1998, 72, 1593–1595. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy level alignment and interfacial electronic structures at organic metal and organic organic interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Gao, W.Y.; Kahn, A. Electronic structure and current injection in zinc phthalocyanine doped with tetrafluorotetracyanoquinodimethane: Interface versus bulk effects. Org. Electron. 2002, 3, 53–63. [Google Scholar] [CrossRef]
- Mitsumoto, R.; Seki, K.; Araki, T.; Ito, E.; Ouchi, Y.; Achiba, Y.; Kikuchi, K.; Yajima, S.; Kawasaki, S.; Okino, F.; et al. Soft X-ray absorption, UV photoemission, and VUV absorption spectroscopic studies of fluorinated fullerenes. J. Electron. Spectrosc. Relat. Phenom. 1996, 78, 453–456. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| V2O5 #1 | |
| Growth time (min) | 5 |
| Gas flow (sccm) | Ar = 30 |
| Sheet resistivity (Ω/sq.) | 48 |
| V2O5 #2 | |
| Growth time (min) | 20 |
| Gas flow (sccm) | Ar = 30 |
| Sheet resistivity (Ω/sq.) | 46 |
| V2O5 #3 | |
| Growth time (min) | 20 |
| Gas flow (sccm) | Ar:O2 = 29:1 |
| Sheet resistivity (Ω/sq.) | 36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.J.; Park, M.G.; Kim, M.S.; Han, J.H.; Cho, S.; Cho, M.-H.; Yi, Y.; Lee, H.; Cho, S.W. Electronic Structure of C60/Zinc Phthalocyanine/V2O5 Interfaces Studied Using Photoemission Spectroscopy for Organic Photovoltaic Applications. Molecules 2018, 23, 449. https://doi.org/10.3390/molecules23020449
Lim CJ, Park MG, Kim MS, Han JH, Cho S, Cho M-H, Yi Y, Lee H, Cho SW. Electronic Structure of C60/Zinc Phthalocyanine/V2O5 Interfaces Studied Using Photoemission Spectroscopy for Organic Photovoltaic Applications. Molecules. 2018; 23(2):449. https://doi.org/10.3390/molecules23020449
Chicago/Turabian StyleLim, Chang Jin, Min Gyu Park, Min Su Kim, Jeong Hwa Han, Soohaeng Cho, Mann-Ho Cho, Yeonjin Yi, Hyunbok Lee, and Sang Wan Cho. 2018. "Electronic Structure of C60/Zinc Phthalocyanine/V2O5 Interfaces Studied Using Photoemission Spectroscopy for Organic Photovoltaic Applications" Molecules 23, no. 2: 449. https://doi.org/10.3390/molecules23020449