1. Introduction
In recent years, cancer incidence rates have risen worldwide. Currently, the health problems caused by cancer treatment are receiving increased attention. Chemotherapy is an important method of therapy in the treatment of malignancies. Different chemotherapeutic agents have different levels of myelosuppression, which may limit the dose that can be used, and can easily result in interruption of chemotherapy. This serious side effect can significantly affect the treatment of malignancies and decrease the quality of a patient’s life. At present, granulocyte colony-stimulating factor is used as adjuvant therapy for the reduction of neutropenia. However, granulocyte colony-stimulating factor also has side effects, including bone pain, and flushing [
1,
2,
3,
4,
5].
In the clinic, extracts from traditional Chinese medicine have received increased attention for their favorable pharmacodynamics effects and low toxicity. In China, burnet root leukopoietic tablets (BRLT) have been widely used due to their beneficial effects on leucopenia caused by tumor-targeting chemotherapy. In previous studies, it was demonstrated that in patients who were given BRLT, the incidence of chemotherapy-induced myelosuppression, was significantly reduced [
6,
7]. BRLT are prepared from
Sanguisorba officinalis L., a traditional Chinese herb that has been used in China for over 3000 years. BRLT have shown beneficial effects in a variety of disease, including infection, inflammatory, cancer, allergic, and diseases of the central nervous system [
8]. Previous studies have demonstrated that
Sanguisorba contains bioactive constituents of saponins and tannins [
9]. Several clinical reports have demonstrated a pre-protective role of
S. officinalis on leucopenia [
10,
11]. Moreover, it has been shown that saponins extracted from this plant reduce myelosuppression and 60 Co-γ-irradiation in mice [
12]. ZGS1 is the main saponin exacted from the famous traditional Chinese medicine [
13], and has the ability to inhibit apoptosis of TF-1 cells, reduce mouse cytokine production, and promote the survival of focal adhesion kinase (FAK) mouse bone marrow nuclear cells in vitro [
14,
15,
16,
17]. In our previous study, we showed that ZGS2 is a metabolite of ZGS1 that protects against the appearance of marrow repression during tumor chemotherapy. Therefore, ZGS1 has been chosen as a novel leucopenia drug and needs further research. Currently, limited information is available on ZGS1 and its metabolite ZGS2 in vivo. Therefore, it is of great importance to investigate these compounds and their pharmacokinetics.
The pharmacokinetic profile of the prototype and their metabolites is a critical estimation of drug candidates. A novel quantitative method for ZGS1 and ZGS2 was established using an Agilent 6460 instrument in this study. This is the first profile study describing the absorption, distribution, and excretion of ZGS1 and ZGS2, and the first time that the pharmacokinetic profile of ZGS1 and its metabolite ZGS2 were investigated. This study provides novel insights for future clinical practice of ZGS1 and ZGS2, which may be promising for the development of ZGS1-based therapeutics in treating leucopenia.
3. Materials and Methods
3.1. Chemical, Reagents, and Materials
ZGS2 and α-hederin (internal standard (IS), HPLC > 98%) were extracted and purified in-house. ZGS1 (HPLC > 98%) was purchased from Raffensi Biotechnology (Chengdu, China). Methanol and acetonitrile (HPLC-MS grade) were purchased from Thermo Fischer Scientific (Fair Lawn, NJ, USA). Formic acid (mass spectrometer grade) was purchased from Aladdin Corporation (Shanghai, China), and Milli-Q ultrapure water was obtained from a Millipore system (Milford, MA, USA).
3.2. LC-MS Conditions
The liquid chromatography (LC) system used was comprised of a binary pump, a constant temperature column chamber, an auto sampler, and a diode array detector (DAD). An XTERRA MS C18 reversed phase column (2.1 × 50 mm, 5 µm) was used for the determination of analytics. The temperature of the column was maintained at 40 °C. The mobile phase A (MA) was water, containing 0.2% formic acid (FA) (v/v) and mobile phase B (MB) contained acetonitrile. The flow velocity was set to 0.4 mL/min. In 6 min, the gradient elution was set to: 0.0–0.1 min, 10% MB; 0.1–1 min, 10% MB; 1–1.5 min, 10–57% MB; 1.5–2.0 min, 57–68% MB; 2.0–3.2 min, 68–75% MB; 3.2–4.0 min, 75–95% MB; 4.0–4.5 min, 95–10% MB; 4.5–6 min, 10% MB. The injection volume was 2 µL. For better stability of the samples, the auto-sampler was kept at a temperature of 4 °C.
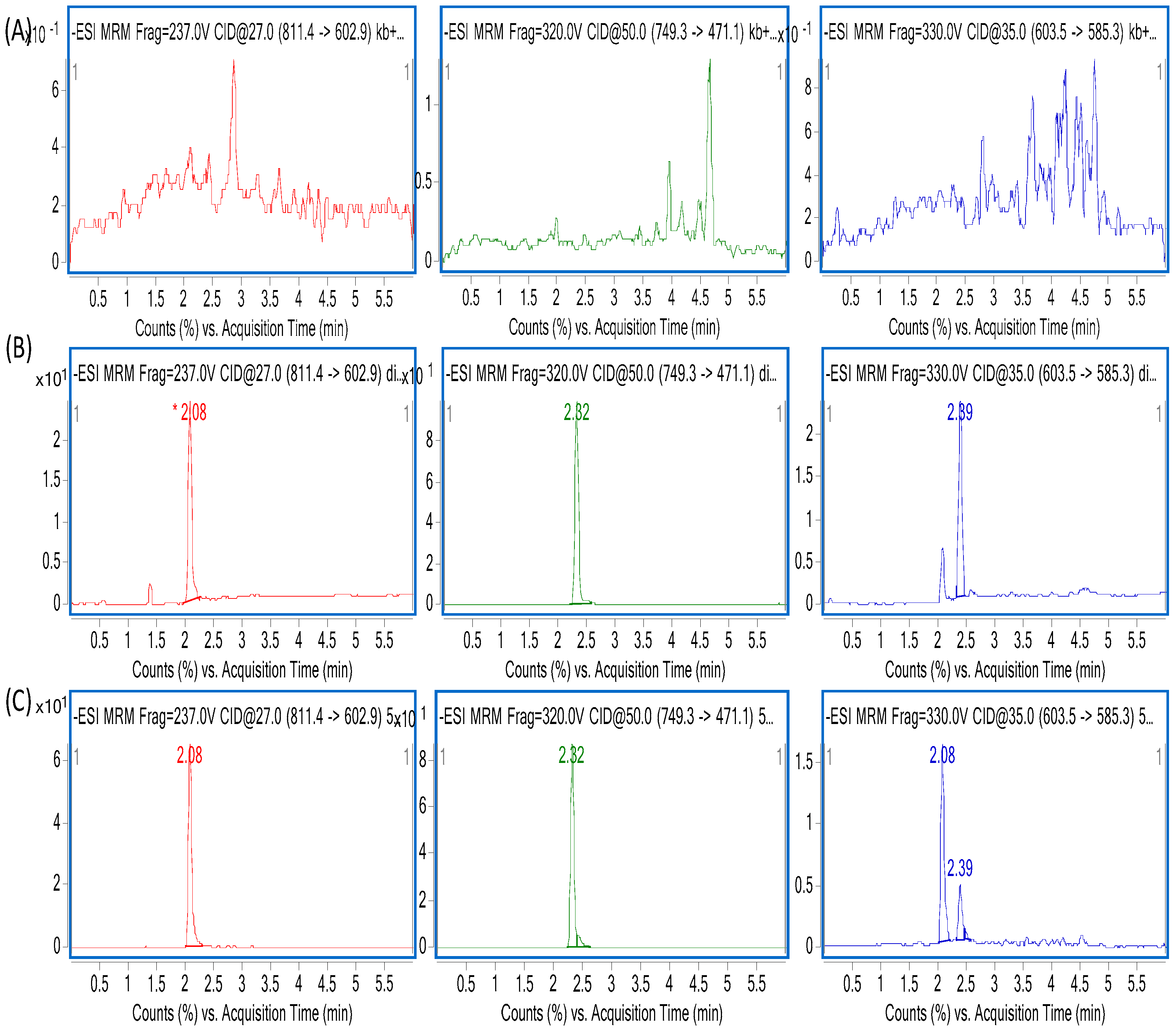
An Agilent 1290 Infinity Rapid Resolution Liquid Chromatography (RRLC) System (Agilent, Lexington, MA, USA), coupled to an Agilent 6460 triple quadrupole mass spectrometer was used for quantitative measurements. Electrospray ionization (ESI) negative and multiple reaction monitoring (MRM) ion mode was used for detection of the ions. The optimal conditions for mass spectrometry were as follows: capillary voltage (CV) 3000 V, gas flow rate 5 L/min, dry gas temperature 300 °C, sheath gas temperature 250 °C, dry flow rate 7 L/min, atomizer pressure 45 psi, and instrument use process using nitrogen. For ZGS2, the precursor-product ion pair was
m/
z 811.4→602.9, for ZGS1,
m/
z 603.4→585.2, and for α-hederin (IS)
m/
z 749.3→471.1 ZGS1, ZGS2, and IS fragments were obtained at −237 V, −330 V, and −320V, respectively. The collision energy (CE) of ZGS1, ZGS2, and IS was −27 eV, −35 eV, and −50 eV, respectively. MassHunter (version B.04) software was used for analysis and data acquisition. Multiple-reaction monitoring was used for further detection. The detailed characteristics are shown in
Table 1.
3.3. Sample Preparation
For preparation of the stock solution (SS), calibration standards, and quality control (QC) samples, ZGS1, ZGS2 as well as α-hederin were dissolved in methanol to a final concentration of 1 mg/mL. Working solutions of calibration standards and QC samples of ZGS1 and ZGS2 were prepared by dilution with methanol (containing 0.1% FA). Similarly, the α-hederin IS was further diluted with methanol (containing 0.1% FA) to a final concentration of 800 ng/mL. For ZGS1 and ZGS2, two stock solutions were prepared, one for the calibration standards, the other for QC samples. Stock solutions were stored at 4 °C.
To achieve a calibration concentration range from 5 to 2000 ng/mL, including 5, 20, 50, 100, 500, 1000, and 2000 ng/mL, stock and working solutions were added to biological samples. For ZGS1 and ZGS2, QC samples of 10, 800, and 1600 ng/mL were prepared. Working solutions for calibration standards of ZGS1 and ZGS2 and QC samples of ZGS1 and ZGS2 were stored at 4 °C for future use.
3.4. Preparation of Biological Samples
A total of 20 μL IS working solution was added to 50 μL plasma, urine, and bile samples. Then, 330 µL methanol (containing 0.1% FA) was added to precipitate the proteins in the samples. After vortex-mixing for 5 min, samples were centrifuged at 12,000 rpm for 5 min at 4 °C. Then, HPLC-MS/MS analysis was used to analyze the supernatant (200 µL). Tissue samples were homogenized in water at a ratio of 1:4 (w/v), while pulverized 0.2 g of fecal material was homogenized with water (1:5, w/v). A total of 50 μL suspension was analyzed.
3.5. Method Validation
The methodology used in this study was performed as per FDA guidelines [
19]. The HPLC-MS/MS analysis for ZGS1 and ZGS2 was validated at a range of 5–2000 ng/mL. Parameters of selectivity, including a lower limit of quantification (LLOQ), matrix effects (ME), precision, accuracy, recovery, stability, and dilution reliability of this method were fully validated.
The specificity of endogenous substances and analytes in rat plasma was determined by determining the chromatograms of plasma samples in six rats. The standard plasma samples used for creating of the calibration curves were assayed. By plotting the ratio of analyte/IS peak area versus the ratio of the nominal concentration, calibration curves were constructed using linear regression (y = ax + b, weighting factor of 1/x2). Acceptance criteria for the LLOQ were ±20% with a correlation coefficient (r2) greater than 0.99. In addition, the acceptable limit of the relative standard deviation (RSD) for each point in the standard curve was ±15%.
Precision and accuracy (Intra-day) assay were evaluated by analyzing replicates (n = 6) at QC levels of 10 (Low quality control, LQC), 800 (Middle quality control, MQC) and 1600 (High quality control, HQC) ng/mL, respectively. Moreover, precision and accuracy (Inter-day) from QCs samples were evaluated at 3 consecutive days. Sample precision and accuracy were assessed by RSD and relative error (RE). The maximum acceptable deviation was ±15%.
To determine the extraction recovery, the peak area ratio extracted from 10 ng/mL (LQC), 800 ng/mL (MQC), and 1600 ng/mL (HQC) QC samples as well as the peak areas that ZGS1 and ZGS2 spiked to the blank sample extracts were calculated at the same concentrations. The ME were determined by matrix factor (MF), which presented the ratio of the ZGS1 and ZGS2 peak response in the presence of matrix ions to the analyte peak response in methanol. Experiments were performed in six replicates at 10 ng/mL, 800 ng/mL and 1600 ng/mL levels.
The stability of ZGS1 and ZGS2 in rat plasma was assessed by analyzing replicates (n = 5) at LQC, MQC, and HQC levels. The short-term stability of the standards and QC samples of ZGS1 and ZGS2 was assessed by evaluating the samples after 24 h at room temperature. The freeze–thaw stability was evaluated by subjecting LQC, MQC, and HQC samples to three freeze–thaw cycles prior to extraction. Samples were stored at −80 °C for 30 days. Then, samples were thawed to room temperature, and analyzed by the approach described above. The analytes were considered stable in plasma when the concentrations were within 85–115% of the initial concentration.
To obtain ZGS1 and ZGS2 samples within the calibration range of 5–2000 ng/mL, samples were diluted using the highest concentration (20,000 ng/L 10-times, the upper limit of quantification (ULOQ)) of the QC sample, which was diluted with plasma.
3.6. Pharmacokinetic Data Analysis
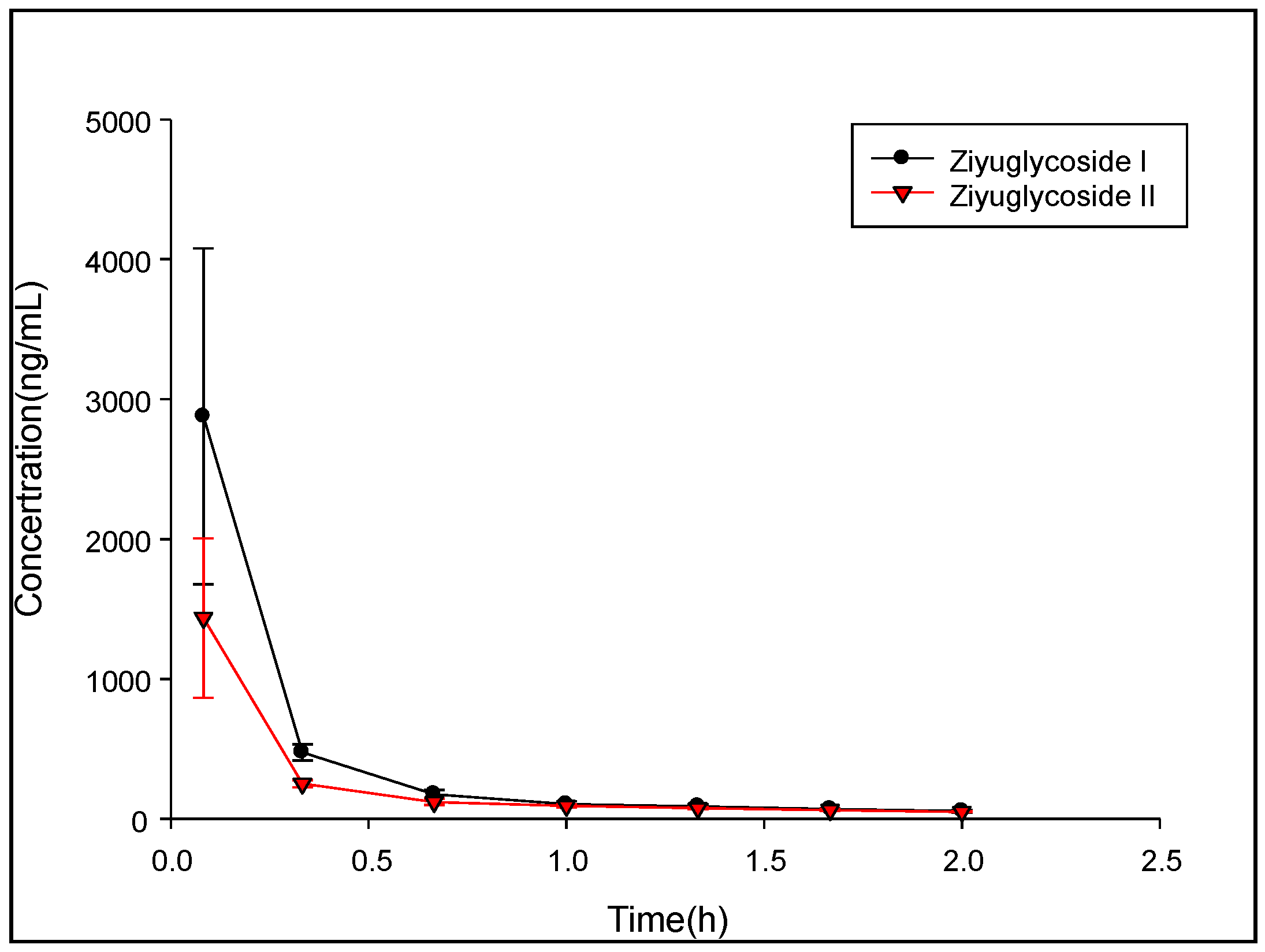
Ten male Sprague-Dawley (SD) rats weighing 150–200 g were purchased from the Hunan Shrek Laboratory Animal (Changsha, China). All experimental procedures involving animals were reviewed and approved by the Animal Care and Use Committee (ACUC) of Jiangxi University of Traditional Chinese Medicine (Nanchang, China) and were in accordance with the Guide for the Care and Use of Laboratory Animals (GCULA). Previous pharmacodynamic studies have shown that in rats, administration of 10 mg/kg ZGS1 has the best beneficial effects. Therefore, we chose to use this dose for our future studies. The general health status of rats was observed daily. Rats were fasted for 12 h but had with free access to water prior to experiments. Rats were randomly divided into two groups, one group received tail vein injection 10 mg/kg ZGS1, the other group received a subcutaneous injection of 10 mg/kg ZGS1. Approximately 0.15 mL of rat blood samples were collected from the orbital venous plexus at the following time points: pre-dose, 0.083, 0.333, 0.666, 1, 1.333, 1.667, 2, 3, 4, and 6 h. Plasma was prepared by centrifugation at 4000× g for 5 min at 4 °C. The supernatant was separated and stored at −40 °C until sample preparation and analysis. Non-compartmental analysis of the data was acquired and processed by using DAS 3.0 software (Chinese Pharmacological Society). Results are expressed as the mean ± standard deviation (SD).
3.7. Tissue Distribution Study
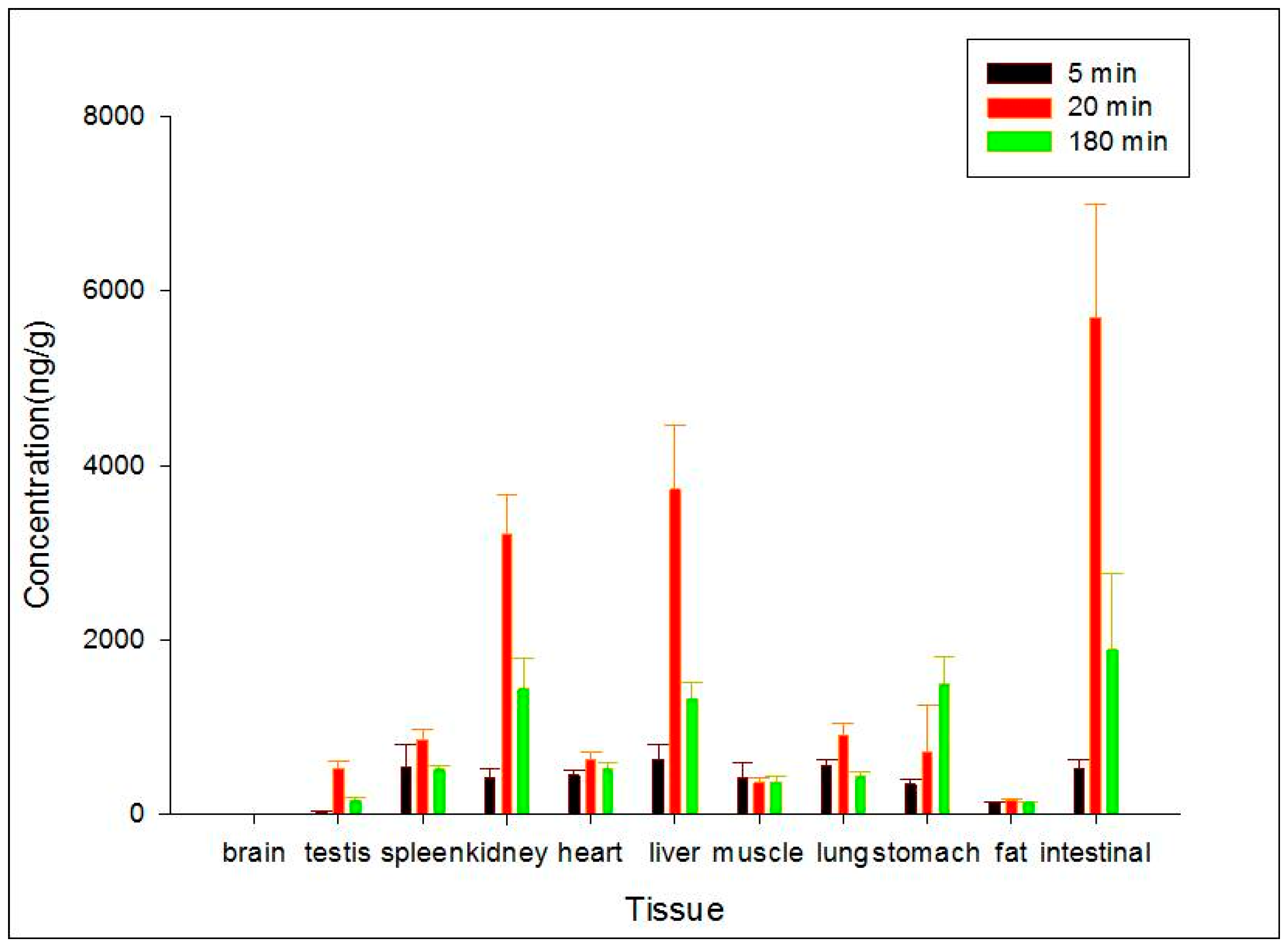
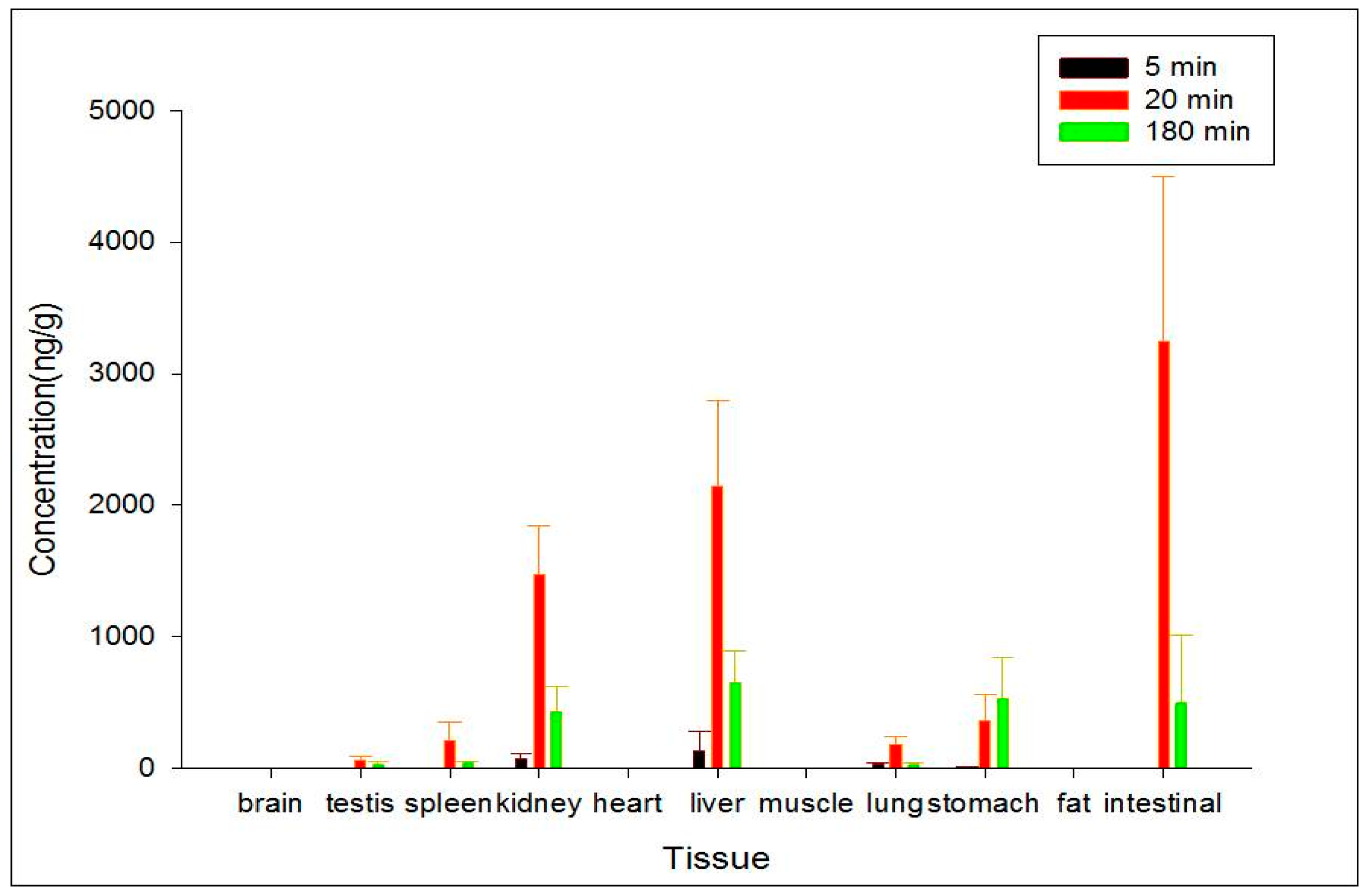
To study the target distribution of ZGS1 and ZGS2, tissue distribution was evaluated. In brief, 15 rats (150–200 g) were randomly divided into three groups. After subcutaneous injection of 10 mg/kg of ZGS1, 11 tissues, including liver, heart, spleen, lung, brain, intestine, stomach, kidney, testis, skeletal muscle, and fat were collected at 5, 20, or 180 min. Tissues were immediately rinsed with normal saline solution, dried, and accurately weighed. Samples were homogenized following the methods described above and stored at −40 °C until LC-MS analysis.
3.8. Excretion Study
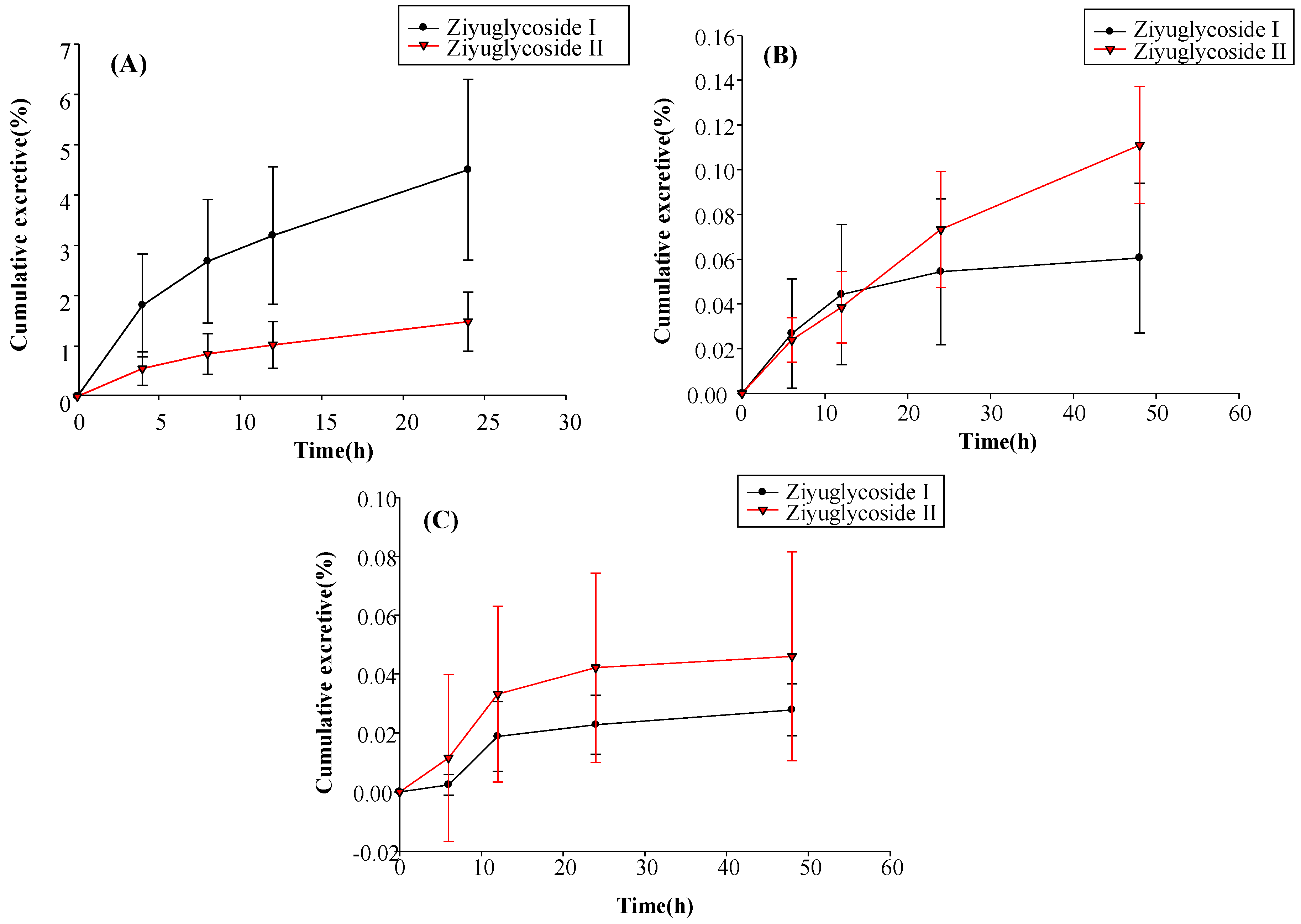
For the excretion study, 12 rats were randomly divided into two groups. One group was used for the urinary and fecal excretion study, whereas the other group was used for evaluating bile excretion. All animals had free access to food and water. Blank samples for urine, feces, and bile were collected before animals were treated. After subcutaneous injection of 10 mg/kg of ZGS1, rats were housed in metabolic cages, and urine and fecal samples were collected at 0–6 h, 6–12 h, 12–24 h, and 24–48 h. Fecal samples were dried at room temperature. After the dry weight of fecal samples and the volume of urine for each collection point were measured, specimens were stored at −40 °C. Rats in the group for bile excretion evaluation were anesthetized with 20% urethane and a cannula was implanted into the bile duct to collect bile. Bile samples were collected at 0–4 h, 4–8 h, 8–12 h, and 12–24 h, and stored at −40 °C.
Investigating the QC samples in the tissue homogenates was sufficient and equivalent to the peak area measured by the plasma samples under the same condition. Therefore, the above findings indicated that the method of plasma samples can be used to determine the content of ZGS1 and ZGS2 in other tissues.
4. Conclusions
In this study, a simple and sensitive HPLC–MS/MS method was developed for the quantification of ZGS1 and ZGS2 in rat biological matrices. The PK, tissue distribution, and excretion of ZGS1 and ZGS2 were evaluated. In this study, we demonstrated that ZGS1 and ZGS2 can rapidly be cleared from rat plasma following tail vein injection and subcutaneous injection of ZGS1. The major organs of ZGS1 and ZGS2 distribution included the intestines, liver, and kidney. The total recovery of ZGS1 in bile, urine, and feces were low, and may potentially be due to in vivo biotransformation. In vivo processing of the ZGS2 metabolite may form the basis of the effect of ZGS1 on leucopenia.
According to the novel drug Research and Development requirement of the FDA and the CFDA (China), the PK profile of the prototype and the metabolite is acritical measure in the search for drug candidates. Several studies have focused on PK profile of prototypes and metabolites [
20,
21,
22,
23]. Previous studies have suggested that, ZGS2 is the main metabolite of ZGS1, and to better understand its beneficial effects in vivo may help to increase the success rate of potential drugs. In our previous study, we indicated that ZGS1 and ZGS2 possessed remarkable activity on myelosuppression mice. To our knowledge, this is the first time that the PK profile of ZGS1 and its metabolite ZGS2 were studied. Therefore, ZGS1 is a potential candidate for the development of novel drugs to treat leucopenia.