Physicochemical Properties of Bovine Serum Albumin-Glucose and Bovine Serum Albumin-Mannose Conjugates Prepared by Pulsed Electric Fields Treatment
Abstract
:1. Introduction
2. Results and Discussion
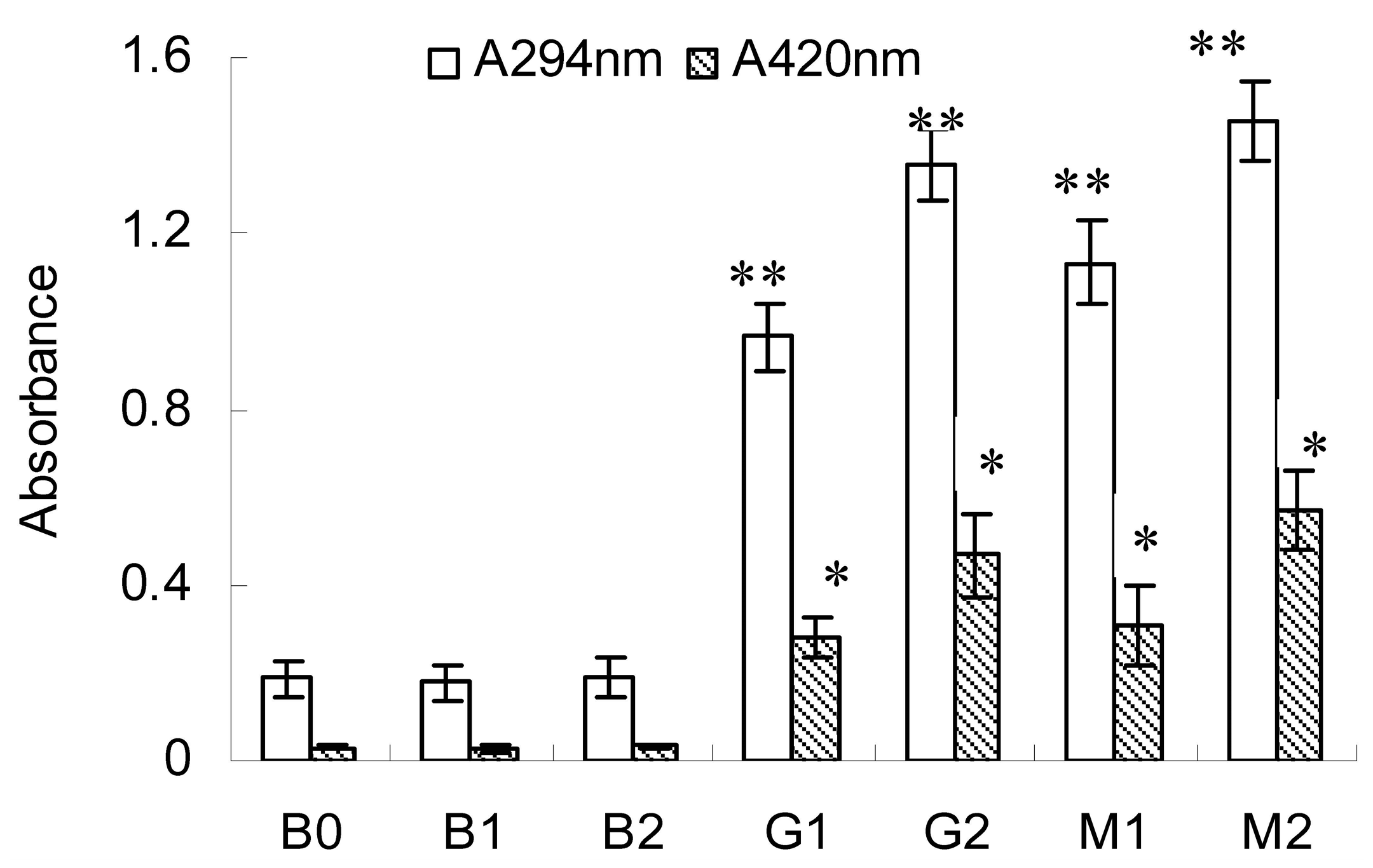
2.1. Changes in A294 and Browning Intensity
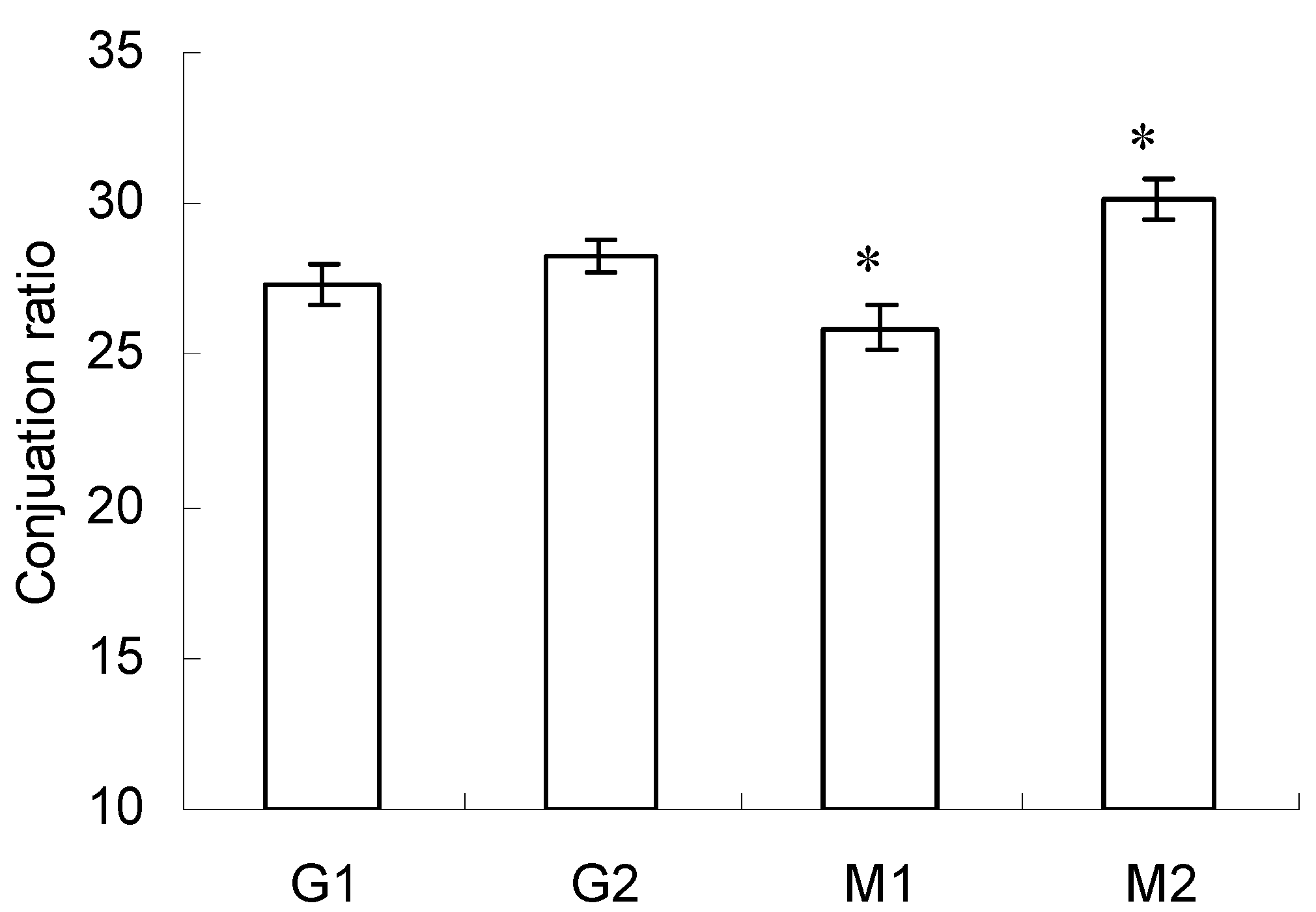
2.2. Conjugation Ratio in Glycated-BSA Conjugates
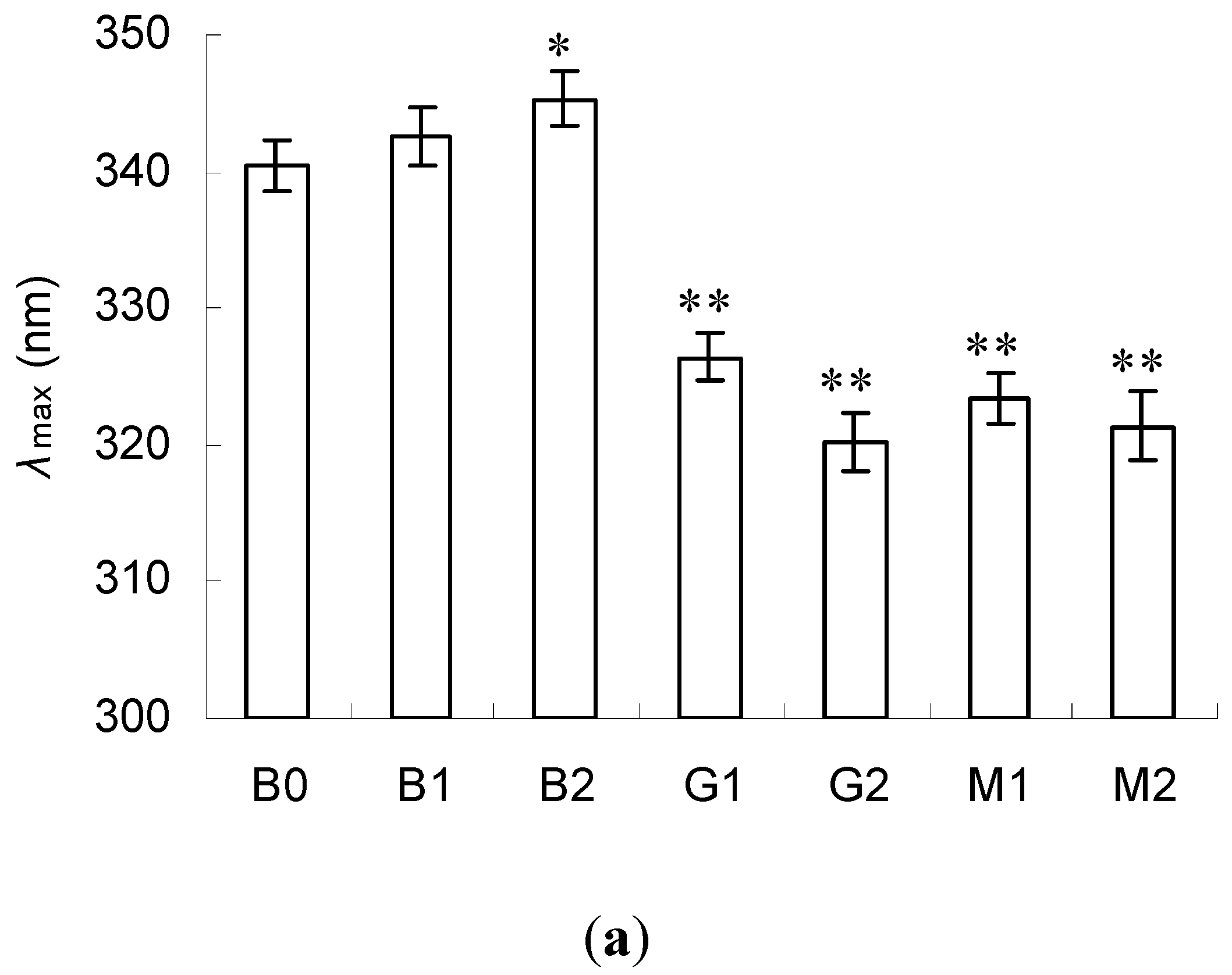
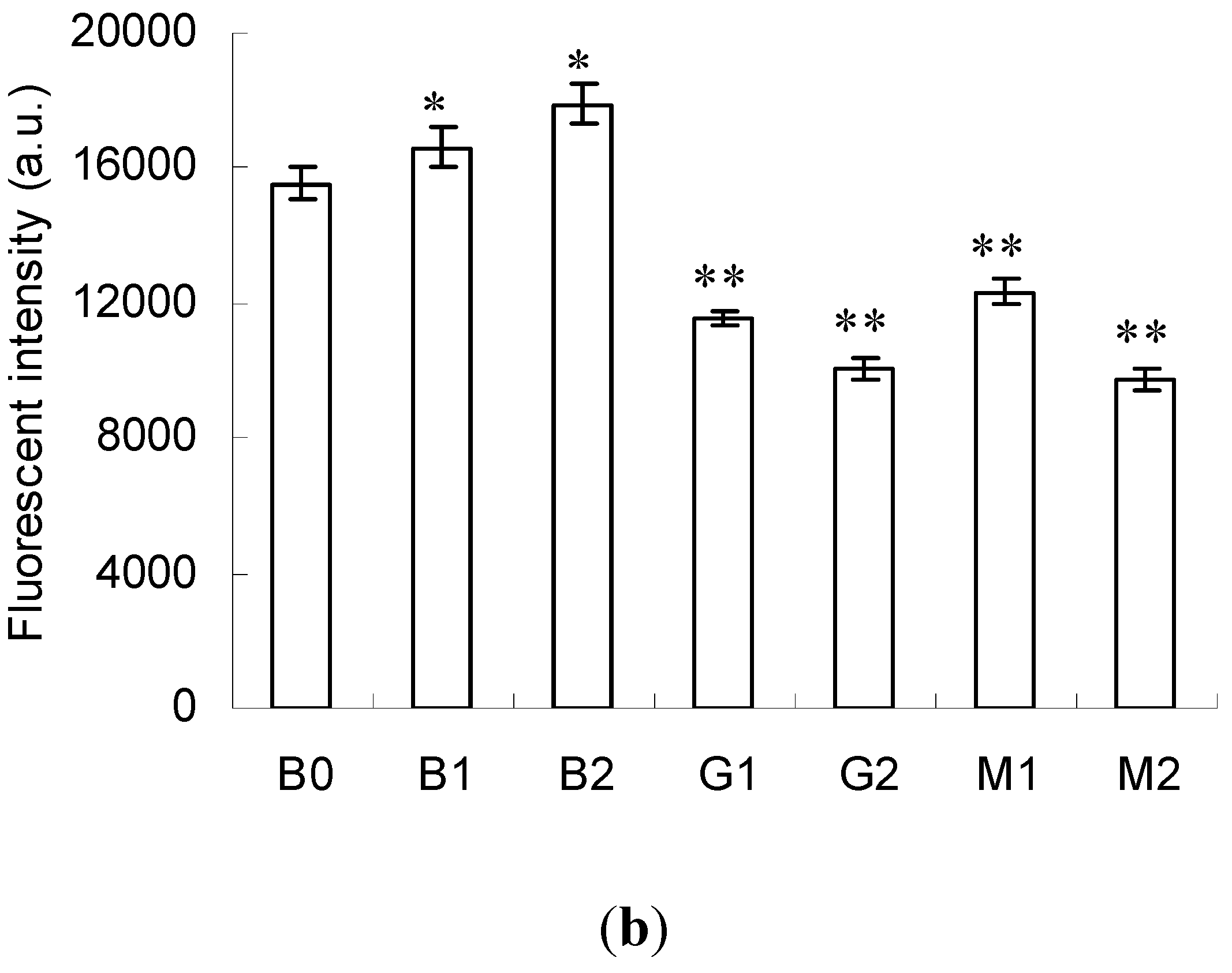
2.3. Emission Fluorescence Spectroscopic Analysis
2.4. Secondary Structure Analysis
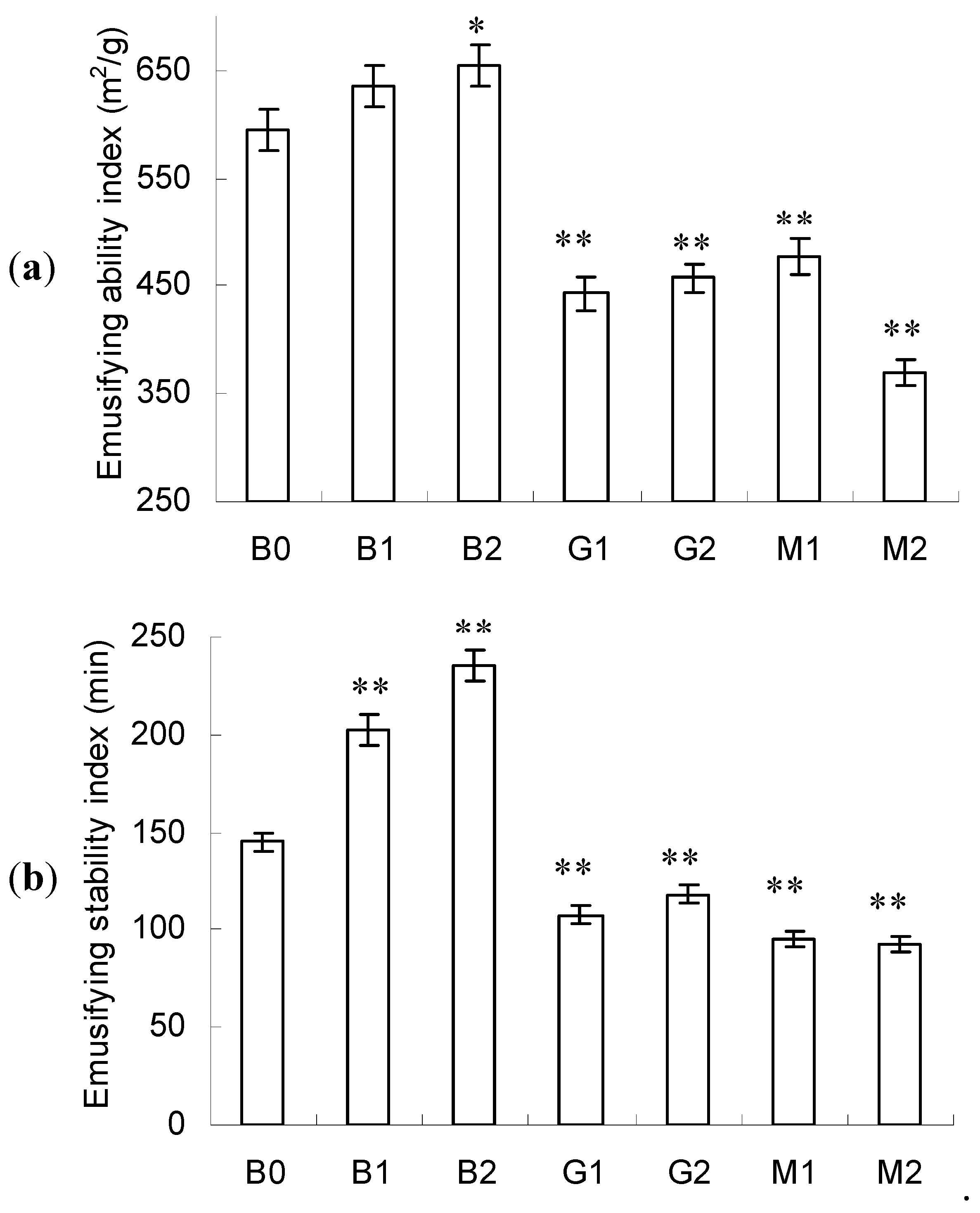
2.5. Emulsifying Activity
2.6. Foaming Activity
3. Materials and Methods
3.1. Chemicals and PEF System
3.2. Preparation of BSA–Glucose and BSA–Mannose Conjugates
3.3. Measurement of Intermediate Products and Browning Value
3.4. Determination of Conjugation Ratio in Glycated-BSA Conjugates
3.5. Measurement of Surface Hydrophobicity
3.6. Circular Dichroism Analysis
3.7. Assay on Physicochemical Properties
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Oliveira, F.C.; Coimbra, J.S.d.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Xia, S.; Li, Y.; Zhao, Q.; Li, J.; Xia, Q.; Zhang, X.; Huang, Q. Probing conformational change of bovine serum albumin–dextran conjugates under controlled dry heating. J. Agric. Food Chem. 2015, 63, 4080–4086. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhong, Q. Thermal aggregation properties of whey protein glycated with various saccharides. Food Hydrocoll. 2013, 32, 87–96. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Martinez-Alvarenga, M.; Martinez-Rodriguez, E.; Garcia-Amezquita, L.; Olivas, G.; Zamudio-Flores, P.; Acosta-Muniz, C.; Sepulveda, D. Effect of maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate—Maltodextrin conjugates. Food Hydrocoll. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Oh, M.-J.; Kim, Y.; Lee, S.H.; Lee, K.-W.; Park, H.-Y. Prediction of CML contents in the maillard reaction products for casein-monosaccharides model. Food Chem. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo-and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- You, J.; Luo, Y.; Shen, H. Functional properties of water-soluble proteins from silver carp (Hypophthalmichthys molitrix) conjugated with five different kinds of sugar. Food Bioprocess Technol. 2013, 6, 3596–3603. [Google Scholar] [CrossRef]
- Jian, W.; He, J.; Sun, Y.; Pang, J. Comparative studies on physicochemical properties of bovine serum albumin-glucose and bovine serum albumin-mannose conjugates formed via Maillard reaction. LWT-Food Sci. Technol. 2016, 69, 358–364. [Google Scholar] [CrossRef]
- Monnier, V.M. The maillard reaction: Good looking to the eyes or a danger to vision? Annu. Rev. Pharmacol. Toxicol. 2017, 57. [Google Scholar]
- Bornhorst, E.R.; Tang, J.; Sablani, S.S.; Barbosa-Cánovas, G.V. Development of model food systems for thermal pasteurization applications based on maillard reaction products. LWT-Food Sci. Technol. 2017, 75, 417–424. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, S.-C.; Hu, C.-C.; Shyu, Y.-S.; Hsu, C.-Y.; Yang, D.-J. Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y. Formation and reduction of acrylamide in maillard reaction: A review based on the current state of knowledge. Crit. Rev. Food Sci. Nutr. 2007, 47, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bremer, P.; Oey, I.; Everett, D. Bacterial inactivation in whole milk using pulsed electric field processing. Int. Dairy J. 2014, 35, 49–56. [Google Scholar] [CrossRef]
- Leong, S.Y.; Richter, L.-K.; Knorr, D.; Oey, I. Feasibility of using pulsed electric field processing to inactivate enzymes and reduce the cutting force of carrot (Daucus carota var. Nantes). Innov. Food Sci. Emerg. Technol. 2014, 26, 159–167. [Google Scholar] [CrossRef]
- Lasekan, O.; Ng, S.; Azeez, S.; Shittu, R.; Teoh, L.; Gholivand, S. Effect of Pulsed Electric Field Processing on Flavor and Color of Liquid Foods. J. Food Proc. Preserv. 2016, 41, e12940. [Google Scholar] [CrossRef]
- Reineke, K.; Schottroff, F.; Meneses, N.; Knorr, D. Sterilization of liquid foods by pulsed electric fields—An innovative ultra-high temperature process. Front. Microbiol. 2015, 6, 400. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.G.; Lin, H.; Han, Z.; Wang, J.; Yu, S.J.; Zeng, X.A.; Liu, Y.Y.; Xu, C.H.; Sun, W.W. Effects of pulsed electric field treatment on a bovine serum albumin-dextran model system, a means of promoting the Maillard reaction. Food Chem. 2010, 123, 275–280. [Google Scholar] [CrossRef]
- Sun, W.-W.; Yu, S.-J.; Zeng, X.-A.; Yang, X.-Q.; Jia, X. Properties of whey protein isolate–dextran conjugate prepared using pulsed electric field. Food Res. Int. 2011, 44, 1052–1058. [Google Scholar] [CrossRef]
- Guan, Y.G.; Wang, J.; Yu, S.J.; Zeng, X.A.; Han, Z.; Liu, Y.Y. A pulsed electric field procedure for promoting maillard reaction in an asparagine–glucose model system. Int. J. Food Sci. Technol. 2010, 45, 1303–1309. [Google Scholar] [CrossRef]
- Chen, H.; Ji, A.; Qiu, S.; Liu, Y.; Zhu, Q.; Yin, L. Covalent conjugation of bovine serum album and sugar beet pectin through Maillard reaction/laccase catalysis to improve the emulsifying properties. Food Hydrocoll. 2016, 76, 173–183. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. J. Food Eng. 2009, 92, 37–43. [Google Scholar] [CrossRef]
- Gaucher, S.P.; Leary, J.A. Stereochemical differentiation of mannose, glucose, galactose, and talose using zinc(II) diethylenetriamine and ESI-ion trap mass spectrometry. Anal. Chem. 1998, 70, 3337. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of Physicochemical and Conformational Properties of Kidney Bean Vicilin (Phaseolin) by Glycation with Glucose: Implications for Structure-Function Relationships of Legume Vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Shen, L. Role of conformational flexibility in the emulsifying properties of bovine serum albumin. J. Agric. Food Chem. 2013, 61, 3097–3110. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-H.; Sun, W.-W.; Yu, S.-J.; Zhao, M.-M. Study on the characteristic of bovine serum albumin-glucose model system, treated by ultrasonic. Food Res. Int. 2010, 43, 2115–2118. [Google Scholar] [CrossRef]
- Rangsansarid, J.; Cheetangdee, N.; Kinoshita, N.; Fukuda, K. Bovine serum albumin-sugar conjugates through the maillard reaction: Effects on interfacial behavior and emulsifying ability. J. Oleo Sci. 2008, 57, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, S.J.; Shin, W.S.; Moon, T.W. Emulsifying properties of bovine serum albumin-galactomannan conjugates. J. Agric. Food Chem. 2003, 51, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Phillips, L. Protein interactions in foams: Protein-GAS phase interactions. Protein Funct. Food Syst. 1994, 9, 181–208. [Google Scholar]
- Sri Harsha, P.S.; Lavelli, V.; Scarafoni, A. Protective ability of phenolics from white grape vinification by-products against structural damage of bovine serum albumin induced by glycation. Food Chem. 2014, 156, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Luck, P.; Davis, J.P. Factors determining the physical properties of protein foams. Food Hydrocoll. 2006, 20, 284–292. [Google Scholar] [CrossRef]
- Höhler, R.; Cohen-Addad, S.; Durian, D.J. Multiple light scattering as a probe of foams and emulsions. Curr. Opin. Colloid Interface Sci. 2014, 19, 242–252. [Google Scholar] [CrossRef]
- Johnson, D.; Hilal, N. Characterisation and quantification of membrane surface properties using atomic force microscopy: A comprehensive review. Desalination 2015, 356, 149–164. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Wang, M.-S. Effect of pulsed electric fields (PEFs) on the pigments extracted from spinach (Spinacia oleracea L.). Innov. Food Sci. Emerg. Technol. 2017, 43, 26–34. [Google Scholar] [CrossRef]
- Guan, Y.-G.; Zhu, S.-M.; Yu, S.-J.; Xu, X.-B.; Zhu, L.-C. Short communication: Possible mechanism for inhibiting the formation of polymers originated from 5-hydroxymethyl-2-furaldehyde by sulfite groups in the dairy thermal process. J. Dairy Sci. 2013, 96, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; García, V.; Rendueles, M.; Díaz, M. Functional properties of isolated porcine blood proteins modified by Maillard’s reaction. Food Hydrocoll. 2012, 28, 267–274. [Google Scholar] [CrossRef]
- Luck, P.J.; Vardhanabhuti, B.; Yong, Y.H.; Laundon, T.; Barbano, D.M.; Foegeding, E.A. Comparison of functional properties of 34% and 80% whey protein and milk serum protein concentrates. J. Dairy Sci. 2013, 96, 5522–5531. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| α-Helix (%) | β-Sheet (%) | β-Turns (%) | Random Coil (%) | |
|---|---|---|---|---|
| B0 | 56.2 ± 0.3 | 7.6 ± 0.4 | 10.8 ± 0.2 | 25.4 ± 0.3 |
| B1 | 55.0 ± 0.4 | 7.3 ± 0.5 | 11.3 ± 0.3 | 26.4 ± 0.2 |
| B2 | 57.4 ± 0.3 | 7.4 ± 0.3 | 9.6 ± 0.4 * | 25.6 ± 0.2 |
| G1 | 45.3 ± 0.4 ** | 9.6 ± 0.3 * | 13.9 ± 0.2 * | 31.2 ± 0.4 * |
| G2 | 42.8 ± 0.3 ** | 10.2 ± 0.3 * | 11.4 ± 0.4 * | 35.6± 0.4 * |
| M1 | 43.2 ± 0.5 ** | 8.9 ± 0.4 * | 12.0 ± 0.3 * | 35.9 ± 0.3 * |
| M2 | 41.6 ± 0.2 ** | 9.5 ± 0.3 * | 12.5 ± 0.4 * | 36.4 ± 0.3 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, W.; Wang, L.; Wu, L.; Sun, Y.-m. Physicochemical Properties of Bovine Serum Albumin-Glucose and Bovine Serum Albumin-Mannose Conjugates Prepared by Pulsed Electric Fields Treatment. Molecules 2018, 23, 570. https://doi.org/10.3390/molecules23030570
Jian W, Wang L, Wu L, Sun Y-m. Physicochemical Properties of Bovine Serum Albumin-Glucose and Bovine Serum Albumin-Mannose Conjugates Prepared by Pulsed Electric Fields Treatment. Molecules. 2018; 23(3):570. https://doi.org/10.3390/molecules23030570
Chicago/Turabian StyleJian, Wenjie, Liangyu Wang, Lanlan Wu, and Yuan-ming Sun. 2018. "Physicochemical Properties of Bovine Serum Albumin-Glucose and Bovine Serum Albumin-Mannose Conjugates Prepared by Pulsed Electric Fields Treatment" Molecules 23, no. 3: 570. https://doi.org/10.3390/molecules23030570




