In Vitro and In Vivo Anti-Osteoarthritis Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside from Polygonum Multiflorum
Abstract
:1. Introduction
2. Results
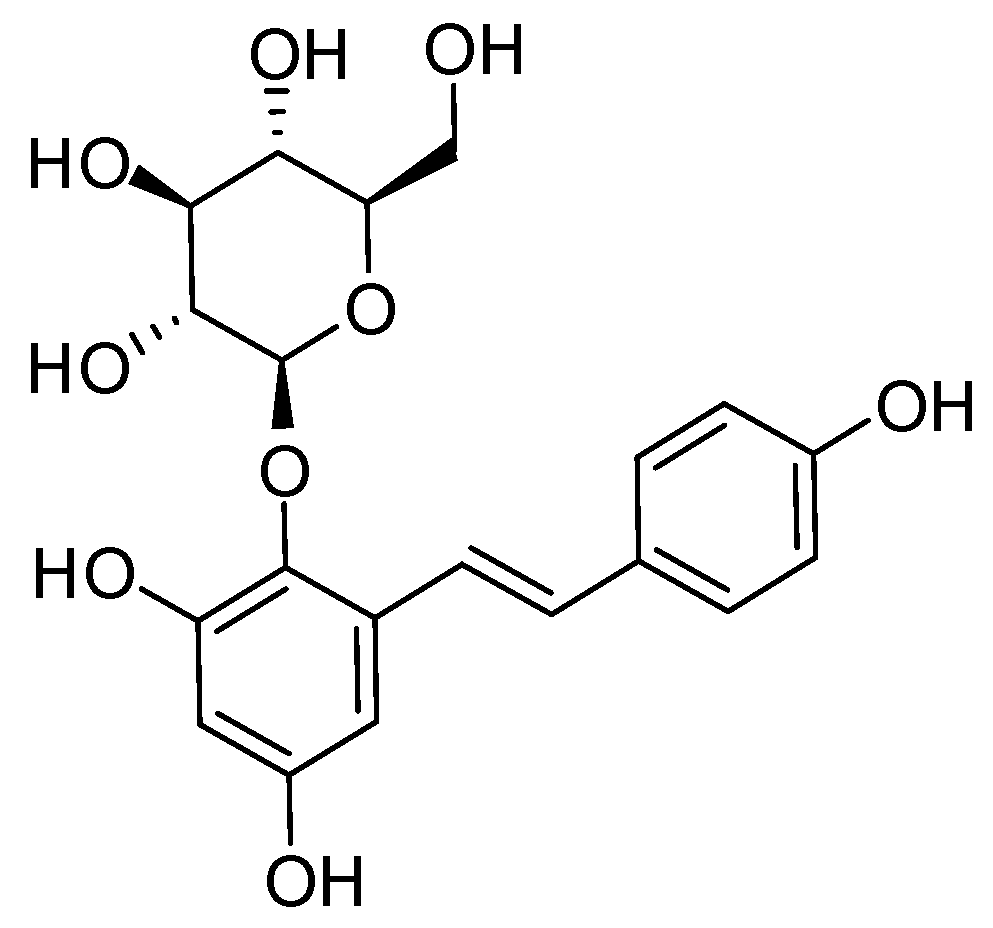
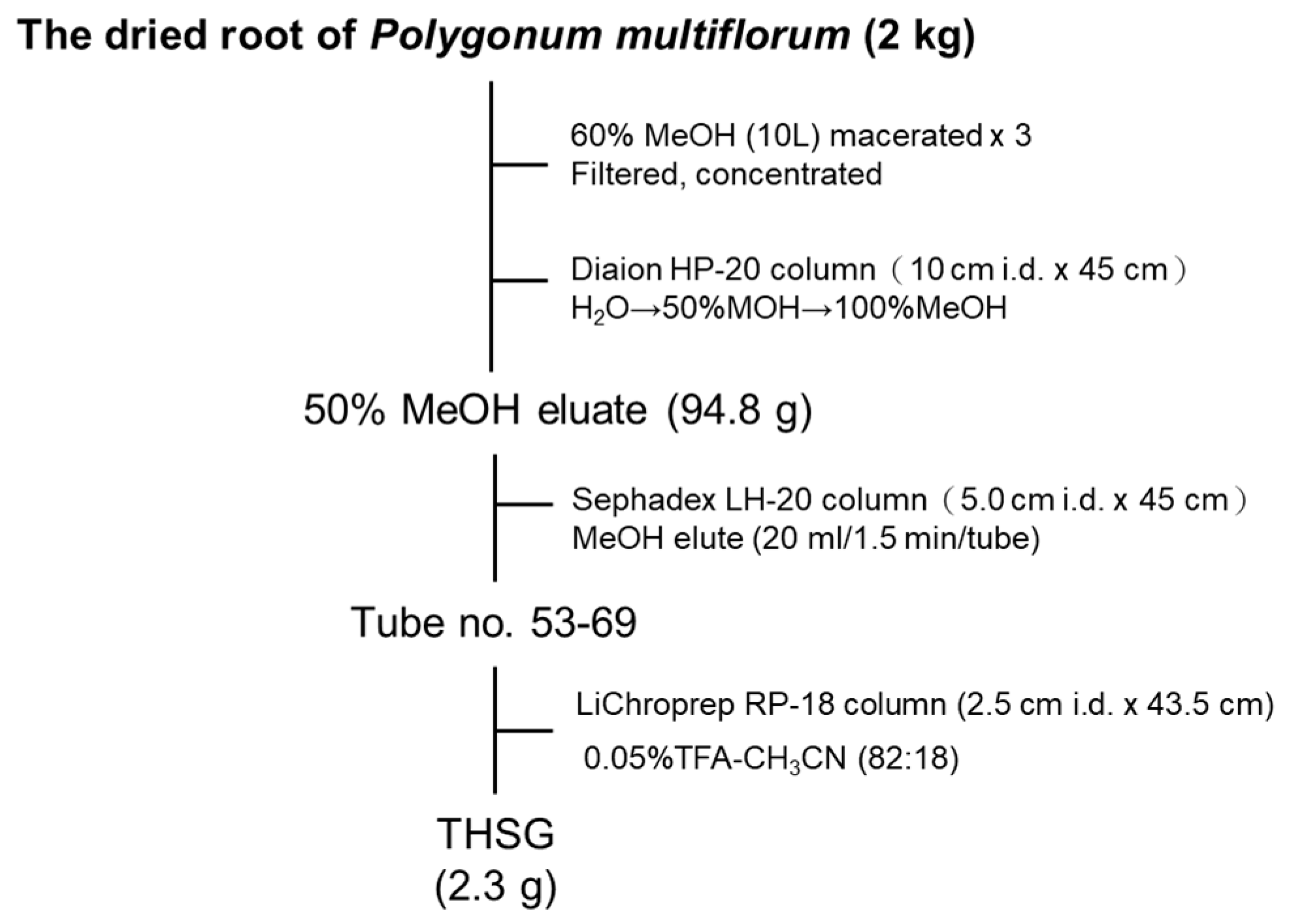
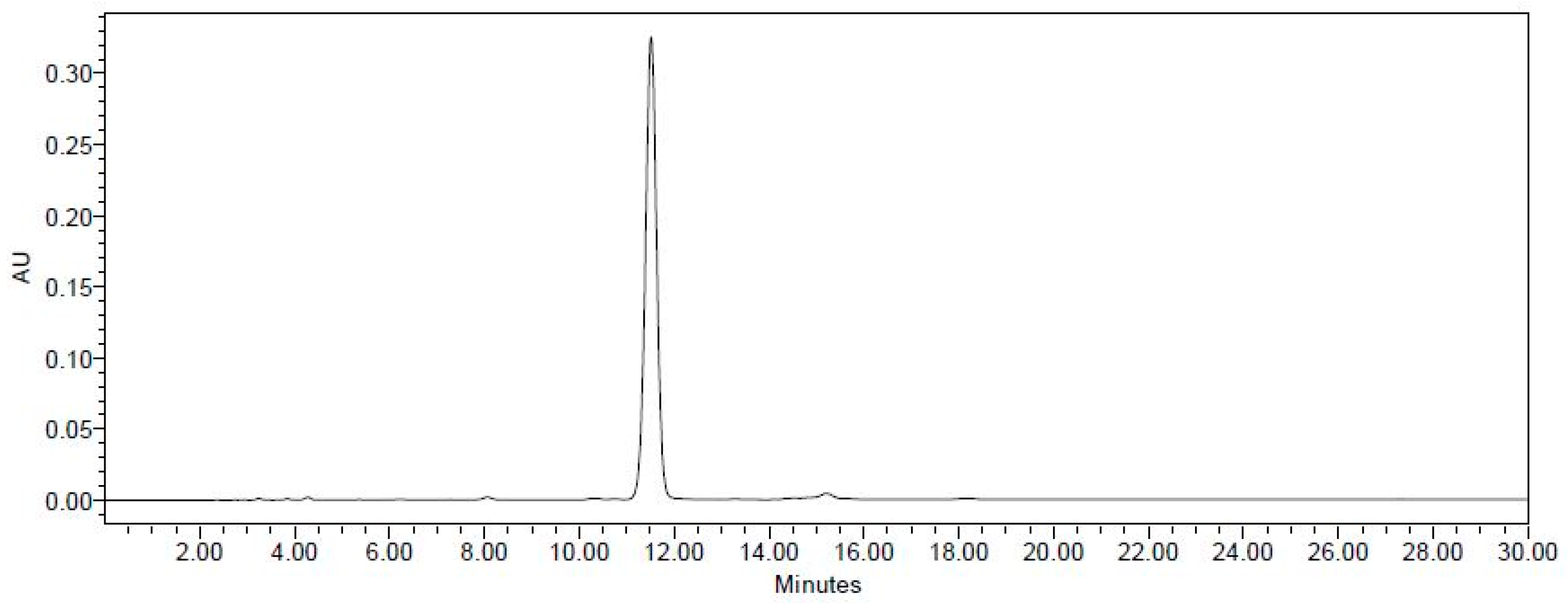
2.1. Establishment of the Isolation Protocol of THSG from PM
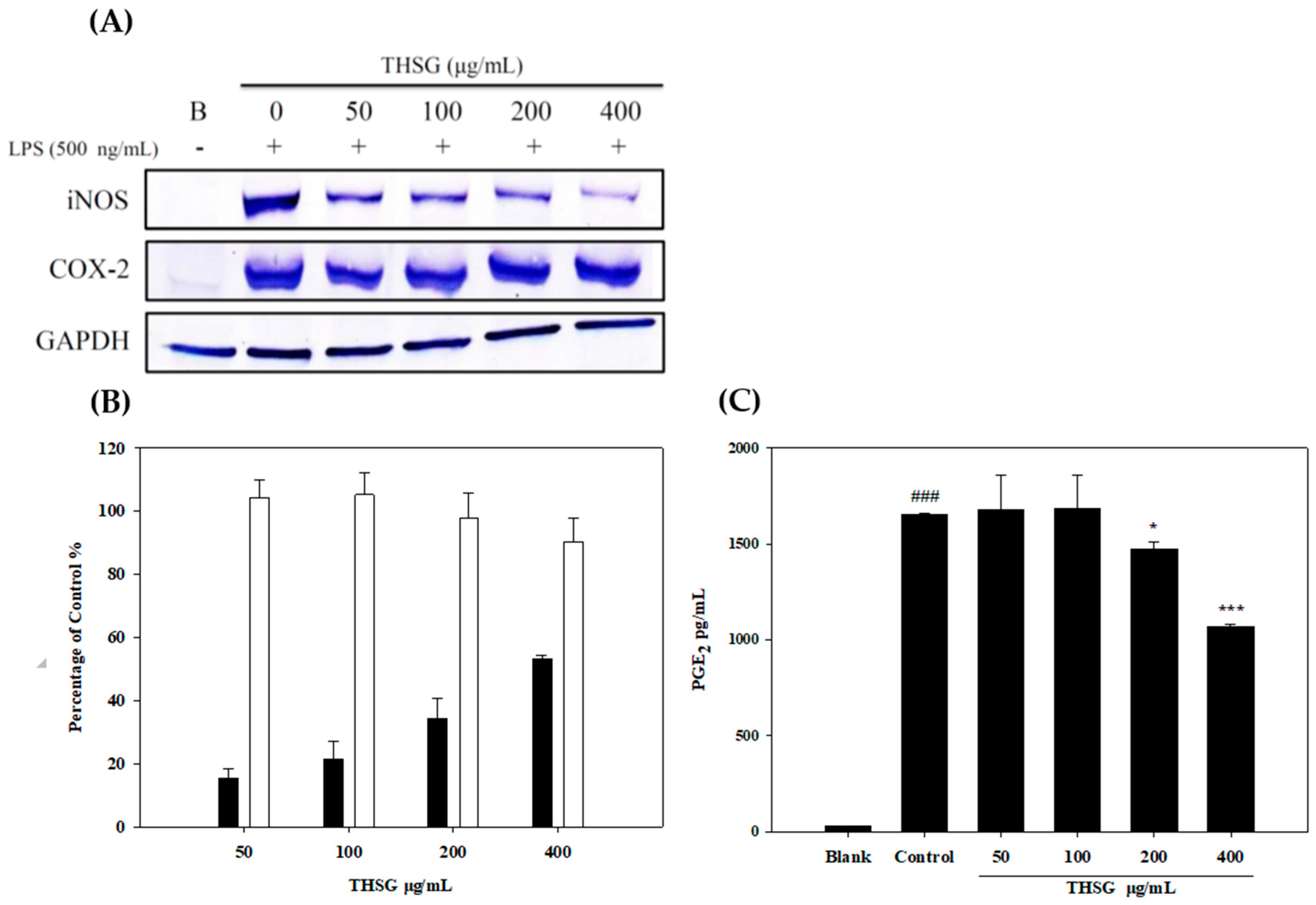
2.2. Anti-Inflammatory Effects of THSG in an In Vitro Assay
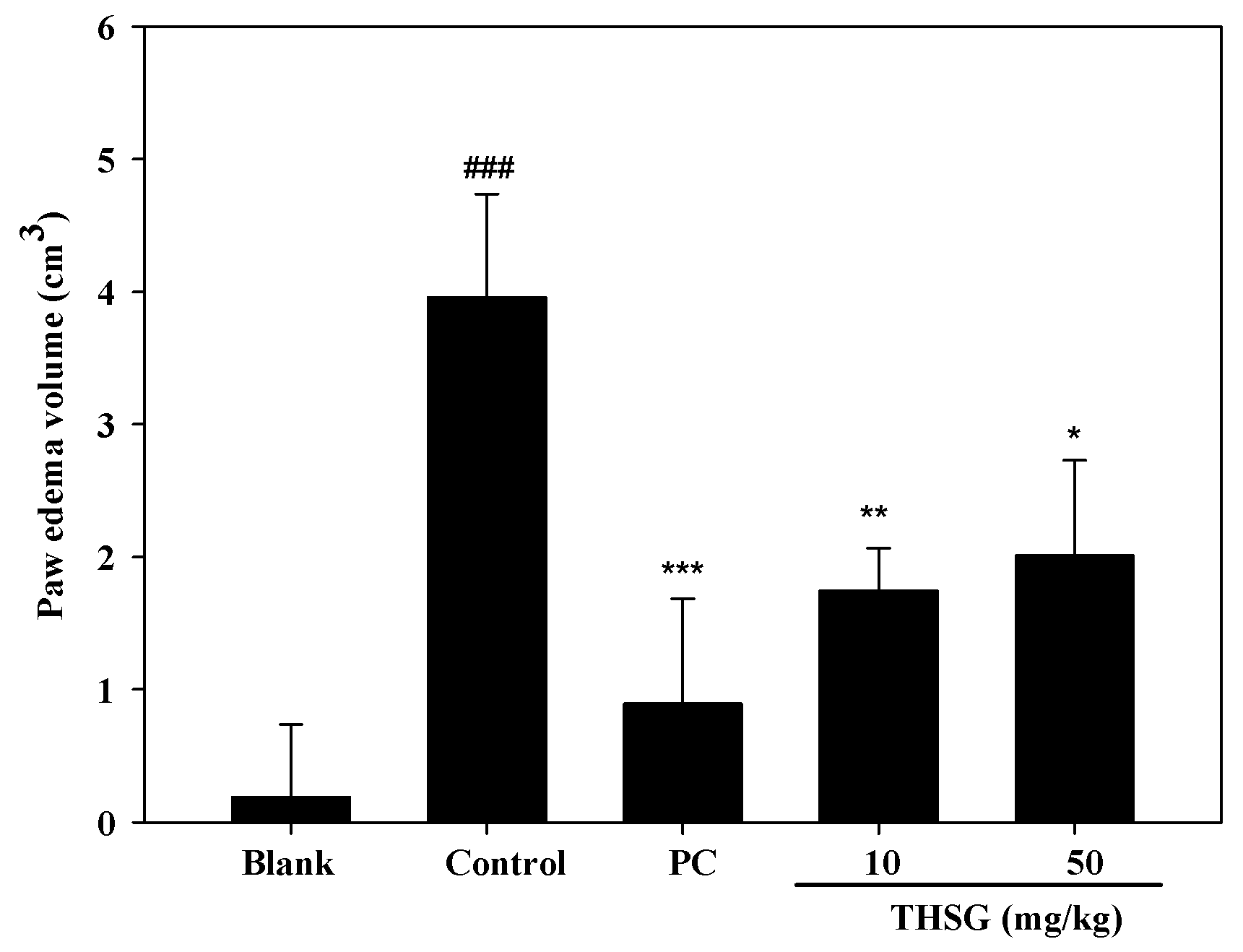
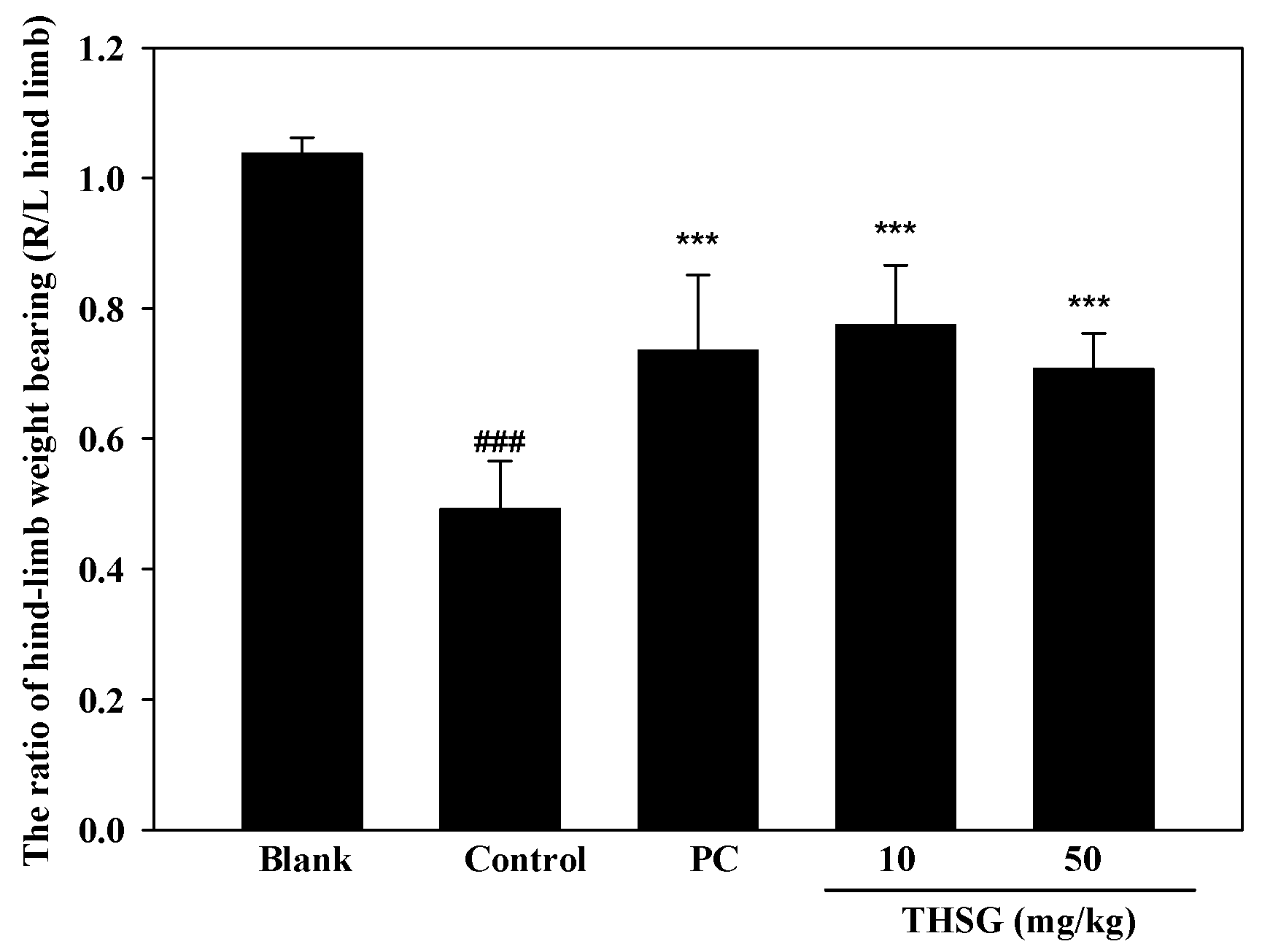
2.3. Anti-OA Effects of THSG in an In Vivo Assay
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction and Isolation of THSG
4.3. Anti-inflammatory In Vitro Assay
4.3.1. Cell Cultures
4.3.2. Primary Chondrocyte Culture
4.3.3. Measurements of iNOS, COX-2, and MMP-13 Protein Expressions
4.3.4. Measurement of NO and PGE2 Production
4.4. Anti-inflammatory In Vivo Assay
4.4.1. Animals
4.4.2. MIA-induced OA Model
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krasnokutsky, S.; Samuels, J.; Abramson, S.B. Osteoarthritis in 2007. Bull. NYU Hosp. Jt. Dis. 2007, 65, 222–228. [Google Scholar] [PubMed]
- Felson, D.T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S. What can we do about osteoarthritis? Arthritis Res. 2000, 2, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.; Krasnokutsky, S. Biomarkers in osteoarthritis. Bull. NYU Hosp. Jt. Dis. 2006, 64, 77–81. [Google Scholar] [PubMed]
- Goldring, S.R.; Goldring, M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal. Interact. 2006, 6, 376–378. [Google Scholar] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.Y.; Huang, K.H.; Lee, C.J.; Tsai, P.W.; Wang, C.C. Antinociceptive and anti-Inflammatory Effects of zerumbone against mono-iodoacetate-induced arthritis. Int. J. Mol. Sci. 2016, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Chabane, N.; Zayed, N.; Afif, H.; Mfuna-Endam, L.; Benderdour, M.; Boileau, C.; Martel-Pelletier, J.; Pelletier, J.P.; Duval, N.; Fahmi, H. Histone deacetylase inhibitors suppress interleukin-1β-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthr. Cartil. 2008, 16, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Eymard, F.; Pigenet, A.; Citadelle, D.; Flouzat-Lachaniette, C.H.; Poignard, A.; Benelli, C.; Berenbaum, F.; Chevalier, X.; Houard, X. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol. 2014, 66, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Liu, Y.; Pan, D.; Wang, Y.; Yang, N.; Xiang, L.; Cai, X.; Feng, Y. Hepatoprotection and hepatotoxicity of Heshouwu, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem. Toxicol. 2017, 108, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.L.; Wang, G.F.; Wang, M.; Ou-Yang, H.Z.; Qi, A.D. Kinetics and mechanism of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glycoside (THSG) degradation in aqueous solutions. J. Pharm. Biomed. Anal. 2011, 55, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L.; Han, T.; Chen, S.; Wang, J. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur. J. Pharmacol. 2008, 578, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Shen, J.F.; Xu, J.Y.; Xiao, J.H.; Wang, J.L. Inhibitory effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside on experimental inflammation and cyclooxygenase 2 activity. J. Asian Nat. Prod. Res. 2007, 9, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Guingamp, C.; Gegout-Pottie, P.; Philippe, L.; Terlain, B.; Netter, P.; Gillet, P. Mono-iodoacetate-induced experimental osteoarthritis: A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997, 40, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, G.-I.; Miwa, N.; Nishioka, I. Stilbene glycoside gallates and proanthocyanidins from Polygonum multiflorum. Phytochemistry 1982, 21, 429–432. [Google Scholar] [CrossRef]
- Sarkar, D.; Saha, P.; Gamre, S.; Bhattacharjee, S.; Hariharan, C.; Ganguly, S.; Sen, R.; Mandal, G.; Chattopadhyay, S.; Majumdar, S.; et al. Antiinflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-kappaB pathway. Int. Immunopharmacol. 2008, 8, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Southan, G.J.; Szabo, C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996, 51, 383–394. [Google Scholar] [CrossRef]
- Liu, R.H.; Hotchkiss, J.H. Potential genotoxicity of chronically elevated nitric oxide: A review. Mutat. Res. 1995, 339, 73–89. [Google Scholar] [CrossRef]
- Ahmad, N.; Chen, L.C.; Gordon, M.A.; Laskin, J.D.; Laskin, D.L. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J. Leukoc. Biol. 2002, 71, 1005–1011. [Google Scholar] [PubMed]
- Chang, Y.C.; Li, P.C.; Chen, B.C.; Chang, M.S.; Wang, J.L.; Chiu, W.T.; Lin, C.H. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cell Signal. 2006, 18, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.; Alvarez-Soria, M.A.; Diez-Ortego, I.; Calvo, E.; Sanchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1β-induced NFkB activation in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Koch, B.; Baum, W.; Burmester, G.R.; Rohwer, P.; Reinke, M.; Zacher, J.; Kirchner, H.; Kalden, J.R. Prostaglandin E2, interleukin 1 and gamma interferon production of mononuclear cells of patients with inflammatory and degenerative joint diseases. Z. Rheumatol. 1988, 48, 194–199. [Google Scholar]
- Sasaki, K.; Hattori, T.; Fujisawa, T.; Takahashi, K.; Inoue, H.; Takigawa, M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J. Biochem. 1998, 123, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R.; Carter, S.D.; Schulze-Tanzil, G.; Shakibaei, M.; Mobasheri, A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet. J. 2003, 166, 140–158. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Matrisian, L.M. Matrix metalloproteinases: A tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Neuhold, L.; Killar, L.; Zhao, W.G.; Sung, M.L.; Warner, L.; Kulik, J.; Turner, J.; Wu, W.; Billinghurst, C.; Meijers, T.; et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Investig. 2001, 107, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Green, C.; Maines, L.W.; Smith, C.D. Experimental osteoarthritis in rats is attenuated by ABC294640, a selective inhibitor of sphingosine kinase-2. Pharmacology 2011, 87, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Cavallo, R.; Dooijes, D.; van Beest, M.; van Es, J.; Loureiro, J.; Ypma, A.; Hursh, D.; Jones, T.; Bejsovec, A.; et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 1997, 88, 789–799. [Google Scholar] [CrossRef]
- Ling, S.; Xu, J.W. Biological activities of 2,3,5,4′-tetrahydroxystilbene-2-O-𝛽-D-glucoside in antiaging and antiaging-related disease treatments. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.P.; Li, S.M.; Liu, Y.; Huang, M.T.; Ho, C.T. Anti-inflammatory effects of trans-2,3,5,4′-tetrahydroxystilbene-2-O-b-glucopyranoside (THSG) from Polygonum multiflorum (PM) and hypoglycemic effect of cis-THSG enriched PM extract. J. Funct. Foods 2017, 34, 1–6. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsieh, S.L.; Wu, C.C.; Huang, C.C.; Leung, W.; Lee, C.T.; Lee, C.H.; Chen, C.Y. Polygonum multiflorum water extract protects against nitric oxide by LPS induced in RAW 264.7 macrophages. Biomed. Res. 2017, 28, 2854–2858. [Google Scholar]
- Wu, X.Q.; Chen, X.Z.; Huang, Q.H.; Fang, D.M.; Li, G.Y.; Zhang, G.L. Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia 2012, 83, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Zheng, M.S.; Lee, Y.K.; Moon, D.C.; Lee, C.S.; Woo, M.H.; Jeong, B.S.; Lee, E.S.; Jahng, Y.D.; Chang, H.W.; et al. A new stilbene glucoside from the roots of Polygonum multiflorum Thunb. Arch. Pharm. Res. 2006, 29, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.H.; Lee, H.H.; Chen, L.G.; Wu, C.H.; Wang, C.C. Effects of three purgative decoctions on inflammatory mediators. J. Ethnopharmacol. 2006, 105, 118–124. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

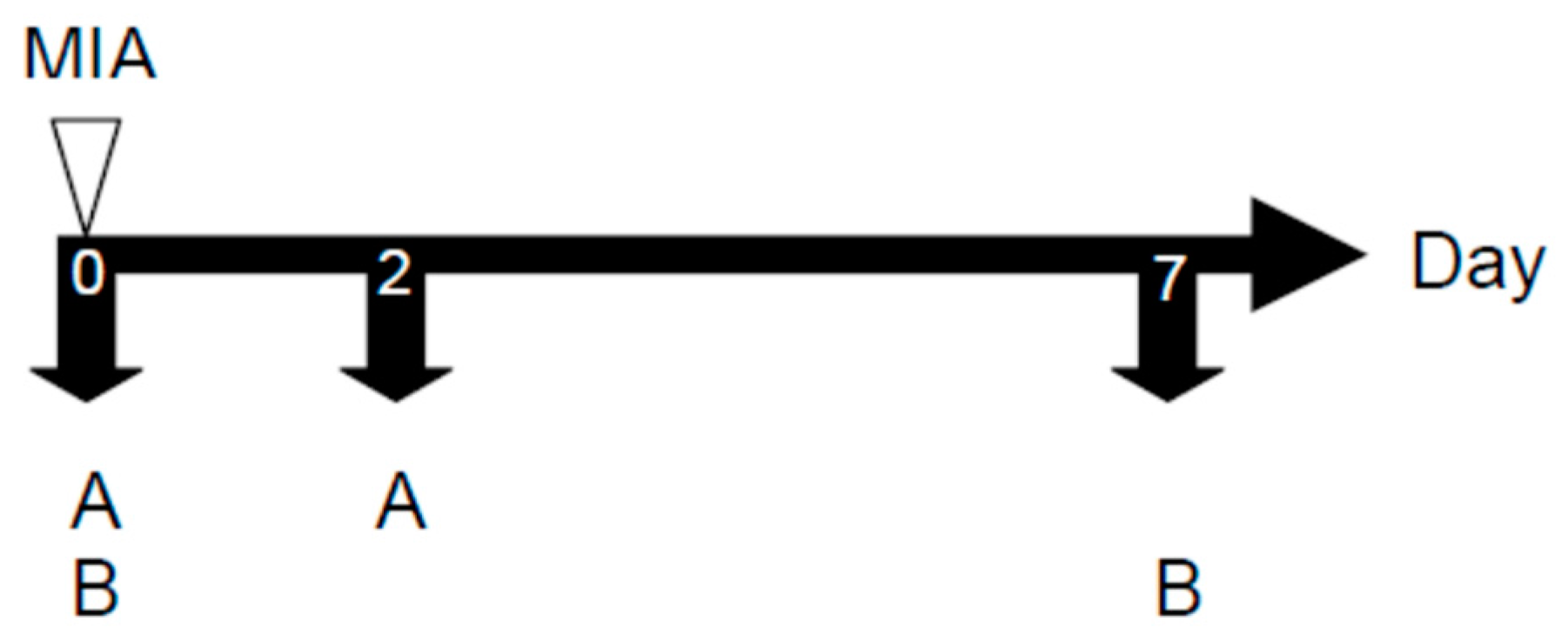
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, P.-W.; Lee, Y.-H.; Chen, L.-G.; Lee, C.-J.; Wang, C.-C. In Vitro and In Vivo Anti-Osteoarthritis Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside from Polygonum Multiflorum. Molecules 2018, 23, 571. https://doi.org/10.3390/molecules23030571
Tsai P-W, Lee Y-H, Chen L-G, Lee C-J, Wang C-C. In Vitro and In Vivo Anti-Osteoarthritis Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside from Polygonum Multiflorum. Molecules. 2018; 23(3):571. https://doi.org/10.3390/molecules23030571
Chicago/Turabian StyleTsai, Po-Wei, Yi-Hui Lee, Lih-Geeng Chen, Chia-Jung Lee, and Ching-Chiung Wang. 2018. "In Vitro and In Vivo Anti-Osteoarthritis Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside from Polygonum Multiflorum" Molecules 23, no. 3: 571. https://doi.org/10.3390/molecules23030571




