Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum
Abstract
:1. Introduction
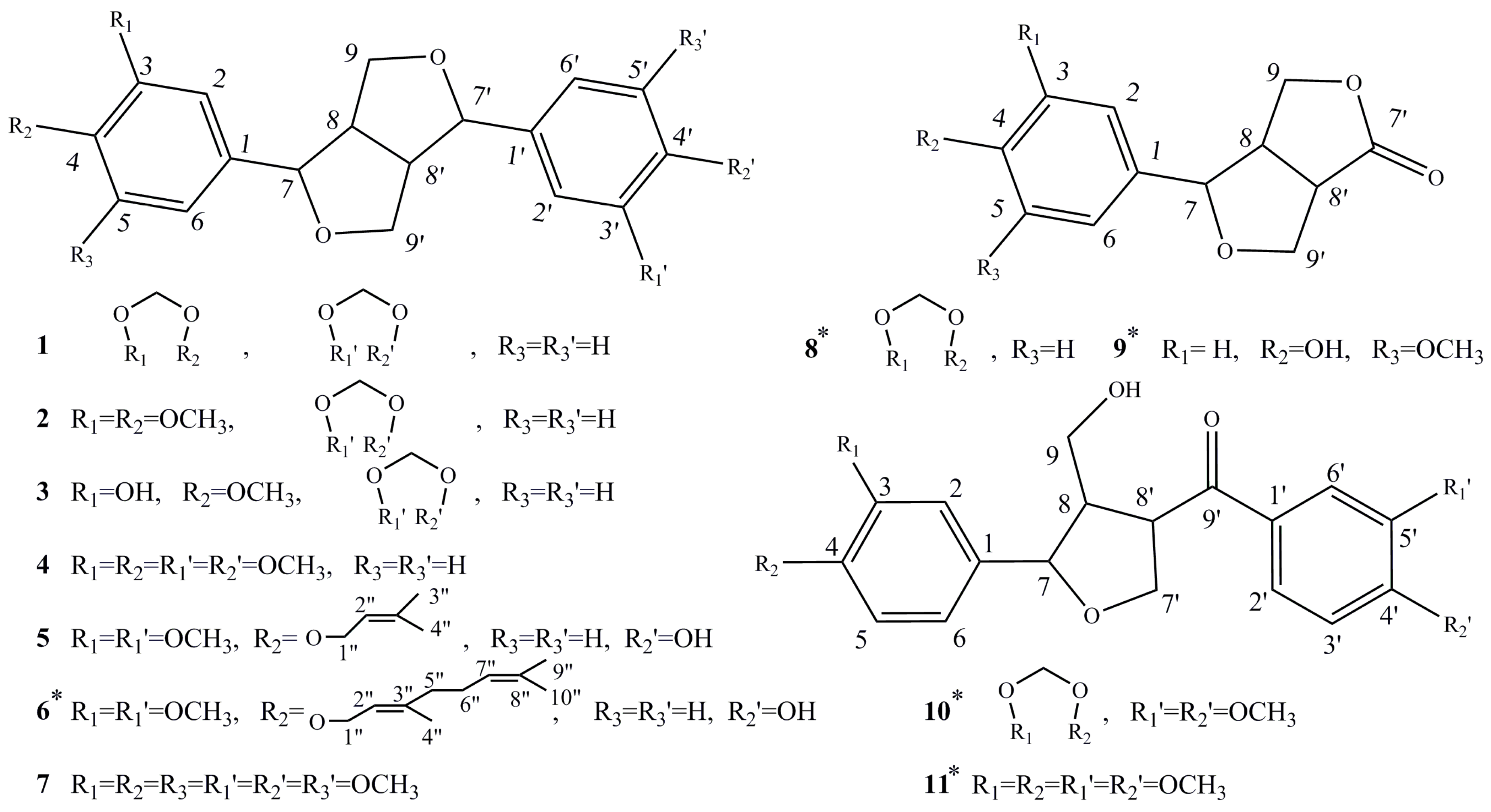
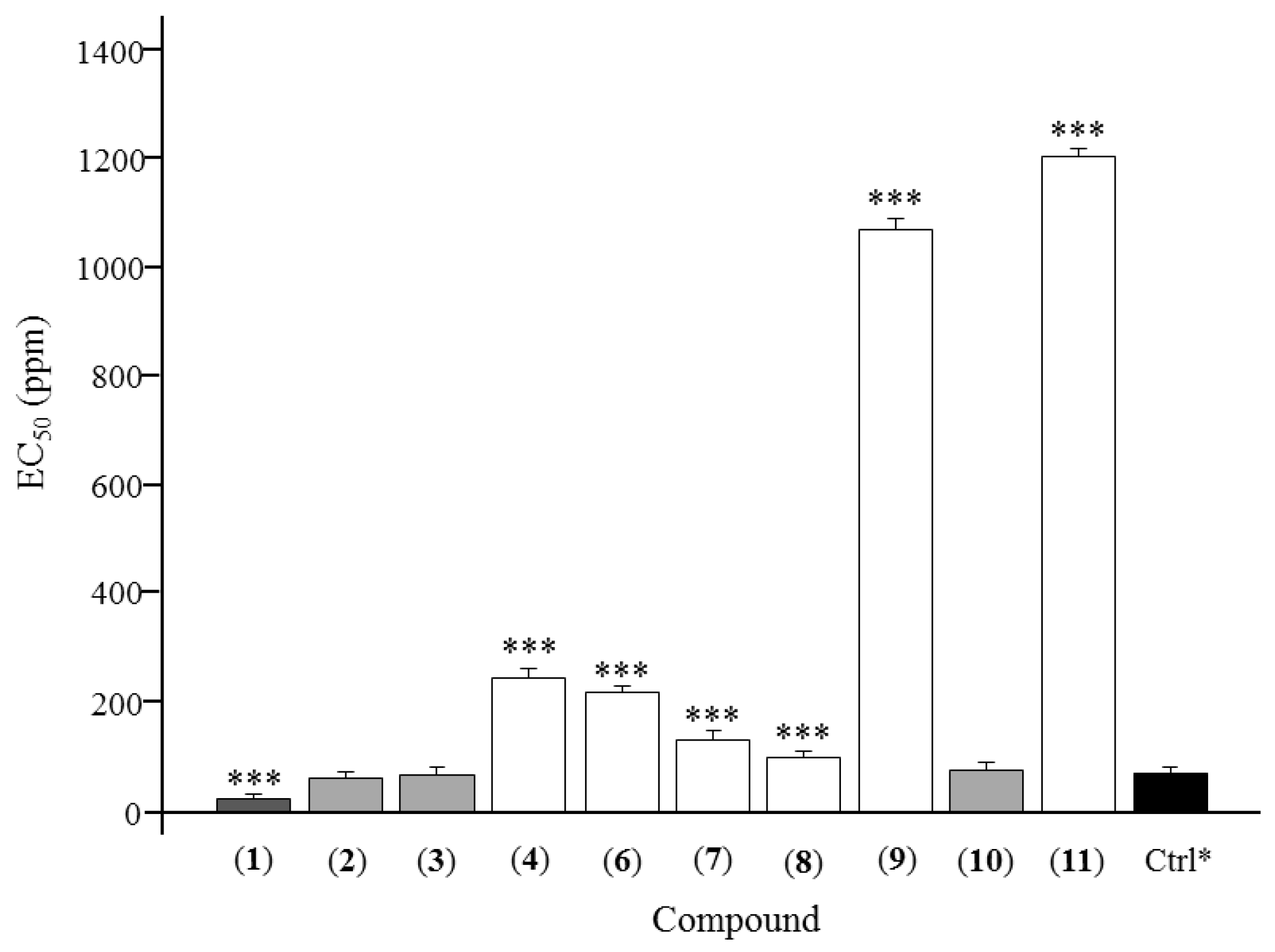
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Material
4.2.1. Plants
4.2.2. Insects
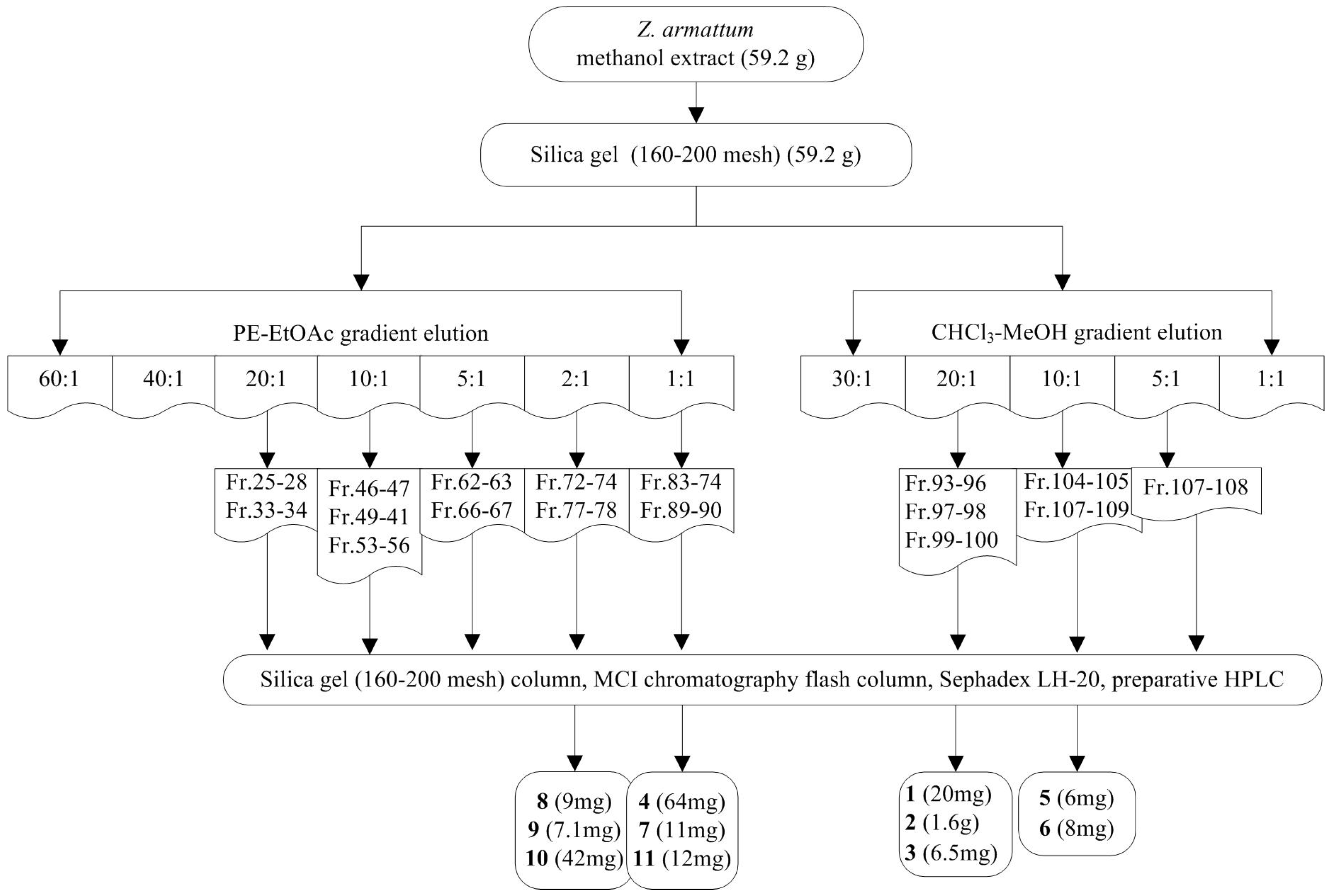
4.3. Extraction and Isolation
4.4. Antifeedant Assay
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hematpoor, A.; Liew, S.Y.; Azirun, M.S.; Awang, K. Insecticidal activity and the mechanism of action of three phenylpropanoids isolated from the roots of Piper sarmentosum Roxb. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, Y.; Shivanandappa, T. A novel natural insecticide molecule for grain protection. Julius-Kühn-Archiv 2010, 910–915. [Google Scholar] [CrossRef]
- Denell, R.; Gibbs, R.; Muzny, D.; Weinstock, G.M.; Attaway, T.; Bell, S.; Buhay, C.J.; Chandrabose, M.N.; Chavez, D.; Clerk-Blankenburg, K.P.; et al. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar]
- Gokhale, C.S.; Traulsen, A.; Joop, G. Social dilemma in the external immune system of the red flour beetle? It is a matter of time. Ecol. Evol. 2017, 7, 6758–6765. [Google Scholar] [CrossRef] [PubMed]
- Kittelmann, S.; Ulrich, J.; Posnien, N.; Bucher, G. Changes in anterior head patterning underlie the evolution of long germ embryogenesis. Dev. Biol. 2013, 374, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Arthur, F.H. Grain protectants: Current status and prospects for the future. J. Stored Prod. Res. 1996, 32, 293–302. [Google Scholar] [CrossRef]
- Adakole, J.A.; Adeyemi, A.F.F. Larvicidal effects of Cymbopogon citratus (lemon grass) extract against Culex quinquefasciatus qularvae (Diptera, culicidae). Int. J. Appl. Environ. Sci. 2012, 7, 187–192. [Google Scholar]
- Tavares, W.S.; Costa, M.A.; Cruz, I.; Silveira, R.D.; Serrao, J.E.; Zanuncio, J.C. Selective effects of natural and synthetic insecticides on mortality of Spodoptera frugiperda (Lepidoptera: Noctuidae) and its predator Eriopis connexa (Coleoptera: Coccinellidae). J. Environ. Sci. Heal 2010, 45, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Us Saqib, Q.N. Evaluation of Zanthoxylum armatum Roxb for in vitro biological activities. J. Tradit. Complement. Med. 2017, 7, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, P.; Zhu, L.; Xie, M. Studies on the chemical constituents of Zanthoxylum armatum DC. Chin. Pharm. 2006, 13, 1035–1036. [Google Scholar]
- Chen, Y.; Hu, Y.; He, H.; Yang, G. Chemical constituents from barks of Zanthoxylum planispinum. Chin. Tradit. Herb. Drugs 2013, 44, 3429–3434. [Google Scholar]
- Agnihotri, S.; Wakode, S.; Ali, M. Chemical constituents isolated from Zanthoxylum armatum stem bark. Chem. Nat. Compd. 2017, 53, 880–882. [Google Scholar] [CrossRef]
- Bhatt, V.; Sharma, S.; Kumar, N.; Singh, B. A new lignan from the Leaves of Zanthoxylum armatum. Nat. Prod. Commun. 2017, 12, 99–100. [Google Scholar]
- Guo, T.; Dai, L.; Tang, X.; Song, T.; Wang, Y.; Zhao, A.; Cao, Y.; Chang, J. Two new phenolic glycosides from the stem of Zanthoxylum armatum DC. Nat. Prod. Res. 2017, 31, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Li, Y. Determination of four lignans in roots, stems and leaves of Zanthoxylum armatum DC by HPLC-DAD with HPLC-ESI-QTOF-MS confirmation. J. Anal. Chem. 2016, 71, 527–533. [Google Scholar] [CrossRef]
- Kumar, V.; Reddy, S.G.E.; Chauhan, U.; Kumar, N.; Singh, B. Chemical composition and larvicidal activity of Zanthoxylum armatum against diamondback moth, Plutella xylostella. Nat. Prod. Res. 2016, 30, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Pu, B.; Jiang, H.; Liu, C. Analysis of fatty acid compositions in Zanthoxylum armatum DC. oil with different extraction methods. J. Nucl. Agric. Sci. 2015, 29, 1323–1328. [Google Scholar]
- Wang, C.; Zhang, W.; You, C.; Guo, S.; Geng, Z.; Fan, L.; Du, S.; Deng, Z.; Wang, Y. Insecticidal Constituents of Essential Oil Derived from Zanthoxylum armatum against Two Stored-Product Insects. J. Oleo Sci. 2015, 64, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pu, B.; Jiang, Y.; Fu, B. Extraction and antioxidant activity in vitro of flavonoids in cold pressed cake of Zanthoxylum armatum DC. Prod. Food Mach. 2017, 33, 137–142. [Google Scholar]
- Xu, L.; Pu, B.; Jiang, Y.; Tan, X.; Wang, S.; Wang, C.; Zhang, L.; Sun, P. Studies on the antioxidatant activities of water extract from Zanthoxylum armatum DC. Prodr. in vitro. Food Ferment. Ind. 2016, 42, 140–143. [Google Scholar]
- Zhou, T.; Pu, B.; Jiang, H.; Liu, C. Study on antibacterial activity and stability of Zanthoxylum armatum DC. Prodr. oil. Sci. Technol. Food Ind. 2015, 36, 106–109. [Google Scholar]
- Alam, F.; Saqib, Q.N.U.; Waheed, A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Meitei, H.T.; Sharma, A.L.; Robinson, A.; Singh, L.S.; Singh, T.R. Anticancer properties and enhancement of therapeutic potential of cisplatin by leaf extract of Zanthoxylum armatum DC. Biol. Res. 2015, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, R.; Verma, P.K.; Singh, R.; Anand, A. Anthelmintic efficacy of aqueous extract of Zanthoxylum armatum DC. seeds against Haemonchus contortus of small ruminants. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2016, 40, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Vitova, M.; Kolouchova, I.; Nedbalova, L.; Dolezalova, J.; Palyzova, A.; Sigler, K. Polydatin and its derivatives inhibit fatty acid desaturases in microorganisms. Eur. J. Lipid Sci. Technol. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Su, G.; Cheng, Y.; Wang, K.; Wang, X.; Wu, B. An unusual tetrahydrofuran lignan from the roots of Zanthoxylum planispinum and the potential anti-inflammatory effects. Chem. Biodivers. 2017, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.G.; Nenadis, N.; Sigalas, M.P. DFT study of radical scavenging activity of sesame oil lignans and selected in vivo metabolites of sesamin. Comput. Theor. Chem. 2016, 1077, 125–132. [Google Scholar] [CrossRef]
- Messiano, G.B.; Vieira, L.; Machado, M.B.; Lopes, L.M.X.; De Bortoli, S.A.; Zukerman-Schpector, J. Evaluation of insecticidal activity of diterpenes and lignans from Aristolochia malmeana against Anticarsia gemmatalis. J. Agric. Food Chem. 2008, 56, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.W.; Suh, W.S.; Subedi, L.; Kim, S.Y.; Kim, A.; Lee, K.R. Bioactive lignan derivatives from the stems of Firmiana simplex. Bioorg. Med. Chem. Lett. 2016, 26, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Han, N.; Liu, Z.; Xu, X.; Zhang, B.; Kadota, S. The in vitro anti-osteoporotic activity of some diarylheptanoids and lignans from the rhizomes of Dioscorea spongiosa. Planta Med. 2008, 74, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, J.; Yang, L.; Ma, J. Analysis and comparison of the volatile oil from different parts of Zanthoxylum ovalifolium var. spinifolium by GC-MS. Chin. Pharm. J. 2004, 39, 502–503. [Google Scholar]
- Jing, Y.; Zhang, Y.; Shang, M.; Yu, J.; Tang, J.; Liu, G.; Li, Y.; Li, X.; Wang, X.; Cai, S. Phenanthrene derivatives from roots and rhizomes of Asarum heterotropoides var. mandshuricum. Fitoterapia 2017, 117, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, T. Studies on the Chemical Constituents of Zanthoxylum armatum DC. and Z. avicenna (Lam.) DC. and Analgesic, Anti-Inflammatory Activites of Z. armatum; Fudan University: Fudan, China, 2011. [Google Scholar]
- Latip, J.; Hartley, T.G.; Waterman, P.G. Lignans and coumarins metabolites from Melicope hayesii. Phytochemistry 1999, 51, 107–110. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Shang, Y.; Yang, G. Two New lignans from the bark of Zanthoxylum planispinum. B Korean Chem. Soc. 2009, 30, 1884–1886. [Google Scholar]
- Ahmed, A.A.; Mahmoud, A.A.; Ali, E.T.; Tzakou, O.; Couladis, M.; Mabry, T.J.; Gati, T.; Toth, G. Two highly oxygenated eudesmanes and 10 lignans from Achillea holosericea. Phytochemistry 2002, 59, 851–856. [Google Scholar] [CrossRef]
- Saladino, R.; Fiani, C.; Crestini, C.; Argyropoulos, D.S.; Marini, S.; Coletta, M. An efficient and stereoselective dearylation of asarinin and sesamin tetrahydrofurofuran lignans to acuminatolide by methyltrioxorhenium/H2O2 and UHP Systems. J. Nat. Prod. 2007, 70, 39–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, R.M.; Alves, C.Q.; David, J.M.; de Rezende, L.C.; Lima, L.S.; David, J.P.; de Queiroz, L.P. Antioxidant activities of isolated compounds from stems of Mimosa invisa mart. ex colla. Quim. Nova 2012, 35, 567–570. [Google Scholar] [CrossRef]
- Yu, H.J.; Chen, C.C.; Shieh, B.J. Two new constituents from the leaves of Magnolia coco. J. Nat. Prod. 1998, 61, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Kim, D.S.; Oh, S.R.; Park, S.H.; Lee, I.S.; Lee, J.J.; Shin, D.H.; Lee, H.K. Magnone A and B, novel anti-PAF tetrahydrofuran lignans from the flower buds of Magnolia fargesii. J. Nat. Prod. 1998, 61, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Du, S.S.; Wang, C.F.; Li, J.; Zhang, H.M.; Liu, Q.Z.; Liu, Z.L.; Deng, Z.W. Antifeedant diterpenoids against Tribolium castaneum from the stems and twigs of Ceriops tagal (Rhizophoraceae). Molecules 2011, 16, 6060–6067. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; You, C.; Yang, K.; Guo, S.; Geng, Z.; Fan, L.; Du, S.; Deng, Z.; Wang, Y. Antifeedant activities of methanol extracts of four Zanthoxylum species and benzophenanthridines from stem bark of Zanthoxylum schinifolium against Tribolium castaneum. Ind. Crop Prod. 2015, 74, 407–411. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | Antifeedant Index (%, Mean ± SD) | EC50 (ppm) | ||||
|---|---|---|---|---|---|---|
| 25 ppm | 74 ppm | 222 ppm | 667 ppm | 2000 ppm | ||
| Asarinin (1) | 49.45 ± 3.78 | 55.51 ± 1.79 | 68.03 ± 4.22 | 70.72 ± 2.83 | 75.83 ± 3.69 | 25.64 ± 3.21 |
| Fargesin (2) | 40.65 ± 4.51 | 52.12 ± 2.31 | 60.71 ± 3.21 | 75.21 ± 1.39 | 81.96 ± 2.93 | 63.24 ± 4.83 |
| Horsfieldin (3) | 34.99 ± 5.32 | 48.91 ± 3.37 | 73.74 ± 3.04 | 73.79 ± 6.01 | 81.12 ± 3.33 | 68.39 ± 5.58 |
| Eudesmin (4) | 35.73 ± 4.37 | 41.28 ± 3.07 | 43.57 ± 2.16 | 55.97 ± 3.46 | 69.82 ± 3.56 | 245.72 ± 3.74 |
| Planispine (5) | - | - | - | - | - | - |
| Planispine B (6) | 29.76 ± 3.16 | 45.95 ± 5.11 | 47.79 ± 3.27 | 58.38 ± 3.17 | 68.64 ± 5.23 | 219.84 ± 4.69 |
| Yangambin (7) | 37.80 ± 3.12 | 49.71 ± 2.61 | 50.82 ± 4.27 | 60.95 ± 3.26 | 66.96 ± 4.16 | 132.50 ± 3.61 |
| Acuminatolide (8) | 39.15 ± 1.21 | 41.36 ± 3.83 | 54.51 ± 2.18 | 77.35 ± 3.65 | 74.76 ± 3.93 | 102.12 ± 2.40 |
| Salicifoliol (9) | 6.82 ± 3.47 | 12.52 ± 2.15 | 24.78 ± 3.17 | 30.46 ± 5.23 | 39.06 ± 4.17 | 1069.20 ± 3.83 |
| Magnolone (10) | 38.93 ± 4.29 | 47.52 ± 1.91 | 62.16 ± 3.93 | 71.94 ± 3.19 | 74.14 ± 3.95 | 78.37 ± 3.44 |
| Magnone A (11) | 21.66 ± 3.91 | 28.99 ± 3.85 | 32.76 ± 4.33 | 43.53 ± 2.77 | 56.60 ± 5.06 | 1203.53 ± 3.95 |
| Toosendanin * | 32.32 ± 2.28 | 52.45 ± 3.27 | 69.52 ± 2.47 | 76.54 ± 3.62 | 86.27 ± 3.51 | 71.69 ± 3.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, Y.; Geng, Z.; Guo, S.; Cao, J.; Zhang, Z.; Pang, X.; Chen, Z.; Du, S.; Deng, Z. Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules 2018, 23, 617. https://doi.org/10.3390/molecules23030617
Zhang W, Wang Y, Geng Z, Guo S, Cao J, Zhang Z, Pang X, Chen Z, Du S, Deng Z. Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules. 2018; 23(3):617. https://doi.org/10.3390/molecules23030617
Chicago/Turabian StyleZhang, Wenjuan, Yang Wang, Zhufeng Geng, Shanshan Guo, Juqin Cao, Zhe Zhang, Xue Pang, Zhenyang Chen, Shushan Du, and Zhiwei Deng. 2018. "Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum" Molecules 23, no. 3: 617. https://doi.org/10.3390/molecules23030617





