Hyperbranched Macromolecules: From Synthesis to Applications
Abstract
:1. Introduction
2. Synthesis of HMs
2.1. Step-Growth Polycondensation
2.2. Self-Condensing Vinyl Polymerization
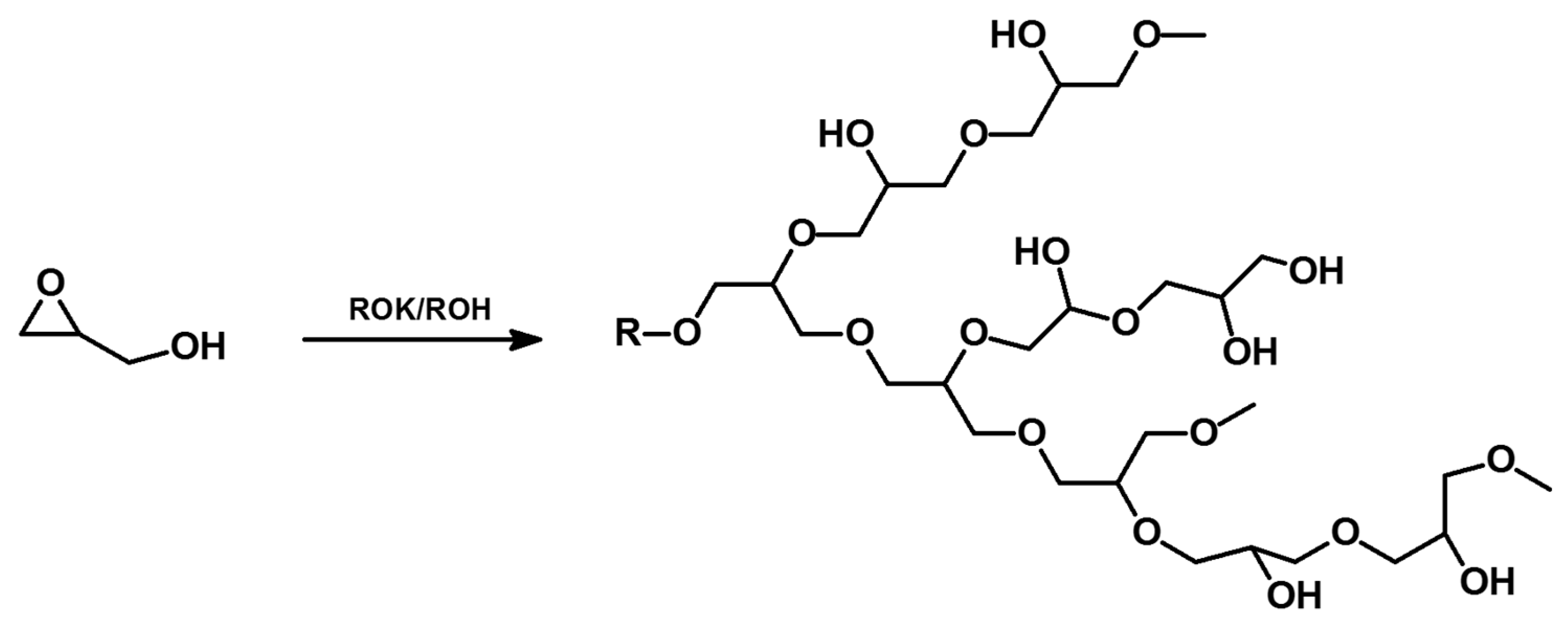
2.3. Ring-Opening Polymerization
2.4. Alternative Routes for HMs
3. Properties of HMs
3.1. Solubility
3.2. Thermal Properties
3.3. Mechanical Properties
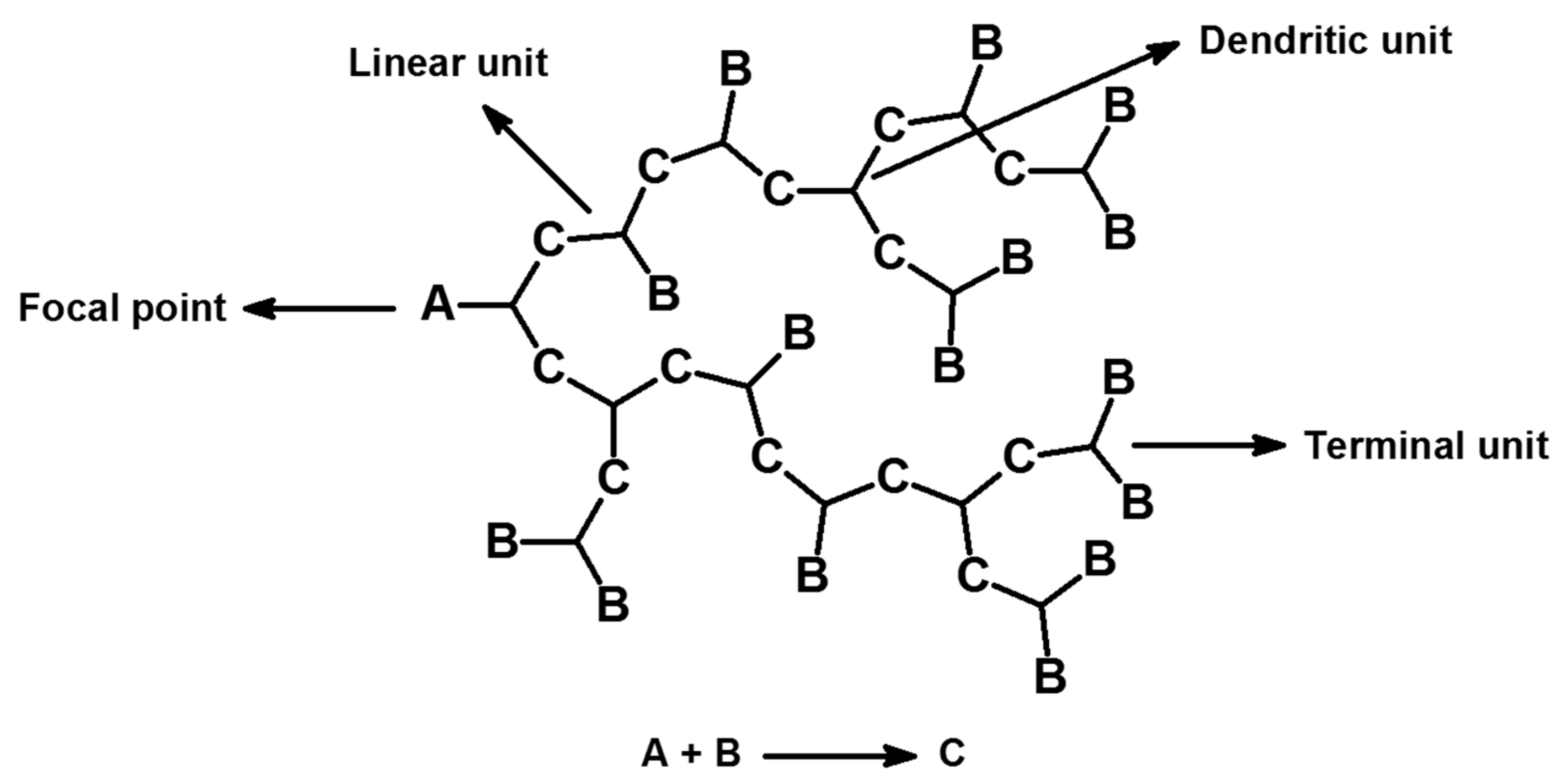
4. Structure of HMs
4.1. Degree of Branching (DB)
4.2. Molecular Weight
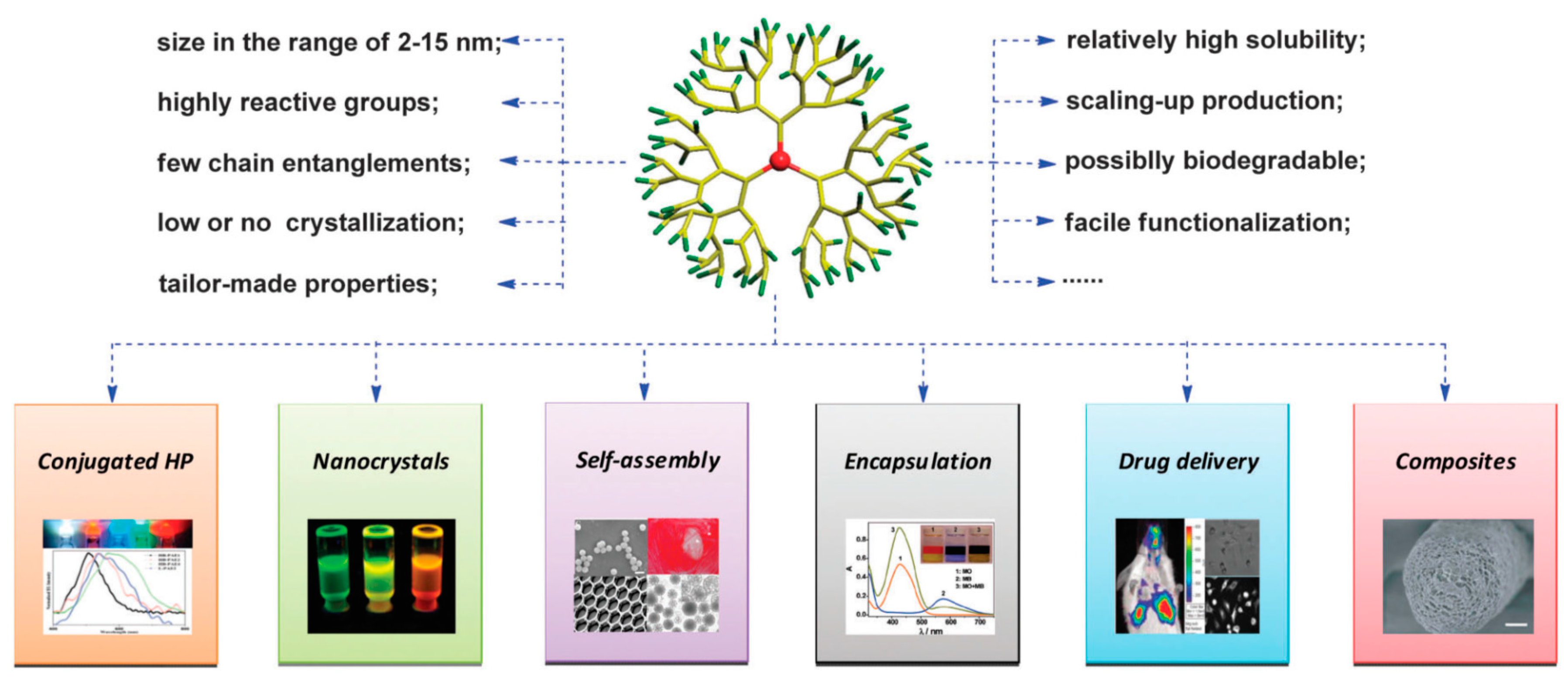
5. Potential Applications of HMs
5.1. Photoelectric Materials
5.2. Stabilizers for Nanocrystals
5.3. Bio-Applications
5.4. Carbon Nanomaterial/HM Nanocomposites
6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 12, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Carminade, A.-M.; Yan, D.; Smith, D.K. Dendrimers and hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 3870–3873. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Zhang, S.; Percec, V.F. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 12, 3900–3923. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tang, R.; Li, Q.; Li, Z. Functional hyperbranched polymers with advanced optical, electrical and magnetic properties. Chem. Soc. Rev. 2015, 12, 3997–4022. [Google Scholar] [CrossRef] [PubMed]
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures—Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Carlmark, A.; Hawker, C.; Hult, A.; Malkoch, M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 2009, 38, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Jikei, M.; Kakimoto, M.-A. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Cook, A.B.; Barbey, R.; Burns, J.A.; Perrier, S. Hyperbranched Polymers with High Degrees of Branching and Low Dispersity Values: Pushing the Limits of Thiol–Yne Chemistry. Macromolecules 2016, 49, 1296–1304. [Google Scholar] [CrossRef]
- Sun, J.; Aly, K.I.; Kuckling, D. A novel one-pot process for the preparation of linear and hyperbranched polycarbonates of various diols and triols using dimethyl carbonate. RSC Adv. 2017, 7, 12550–12560. [Google Scholar] [CrossRef]
- Kim, Y.H.; Webster, O.W. Hyperbranched polyphenylenes. Macromolecules 1992, 25, 5561–5572. [Google Scholar] [CrossRef]
- Aydogan, C.; Yilmaz, G.; Yagci, Y.; Morgenroth, F. Synthesis of Hyperbranched Polymers by Photoinduced Metal-Free ATRP. Macromolecules 2017, 50, 9115–9120. [Google Scholar] [CrossRef]
- Khalyavina, A.; Schallausky, F.; Komber, H.; Samman, M.A.; Radke, W.; Lederer, A. Aromatic–Aliphatic Polyesters with Tailored Degree of Branching Based on AB/AB2 and ABB*/AB2 Monomers. Macromolecules 2010, 43, 3268–3276. [Google Scholar] [CrossRef]
- Xue, Z.; Finke, A.D.; Moore, J.S. Synthesis of Hyperbranched Poly(m-phenylene)s via Suzuki Polycondensation of a Branched AB2 Monomer. Macromolecules 2010, 43, 9277–9282. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Wang, J.-S.; Hewitt, J.M.; Lenhart, W.C.; Mourey, T.H. Acid chloride-functionalized hyperbranched polyester for facile and quantitative chain-end modification: One-pot synthesis and structure characterization. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2855–2867. [Google Scholar] [CrossRef]
- Mahdavi, H.; Shahalizade, T. Preparation, characterization and performance study of cellulose acetate membranes modified by aliphatic hyperbranched polyester. J. Membr. Sci. 2015, 473, 256–266. [Google Scholar] [CrossRef]
- Niu, S.; Yan, H.; Chen, Z.; Li, S.; Xu, P.; Zhi, X. Unanticipated bright blue fluorescence produced from novel hyperbranched polysiloxanes carrying unconjugated carbon–carbon double bonds and hydroxyl groups. Polym. Chem. 2016, 7, 3747–3755. [Google Scholar] [CrossRef]
- Ye, L.; Letchford, K.; Heller, M.; Liggins, R.; Guan, D.; Kizhakkedathu, J.N.; Brooks, D.E.; Jackson, J.K.; Burt, H.M. Synthesis and Characterization of Carboxylic Acid Conjugated, Hydrophobically Derivatized, Hyperbranched Polyglycerols as Nanoparticulate Drug Carriers for Cisplatin. Biomacromolecules 2011, 12, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Perala, S.K.; Ramakrishnan, S. Effect of Spacer Stiffness on the Properties of Hyperbranched Polymers. Macromolecules 2017, 50, 8536–8543. [Google Scholar] [CrossRef]
- Fréchet, J.M.J.; Henmi, M.; Gitsov, I.; Aoshima, S.; Leduc, M.R.; Grubbs, R.B. Self-Condensing Vinyl Polymerization: An Approach to Dendritic Materials. Science 1995, 269, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, T.; Segawa, Y.; Sinananwanich, W.; Ueda, M. Synthesis of hyperbranched polymers with controlled degree of branching. Polym. J. 2011, 44, 14–29. [Google Scholar] [CrossRef]
- Satoh, Y.; Miyachi, K.; Matsuno, H.; Isono, T.; Tajima, K.; kakuchi, T.; Satoh, T. Synthesis of Well-Defined Amphiphilic Star-Block and Miktoarm Star Copolyethers via t-Bu-P4-Catalyzed Ring-Opening Polymerization of Glycidyl Ethers. Macromolecules 2016, 49, 499–509. [Google Scholar] [CrossRef]
- Rannard, S.; Davis, N.; McFarland, H. Synthesis of dendritic polyamides using novel selective chemistry. Polym. Int. 2000, 49, 1002–1006. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Oh, S.-J.; Lee, H.-J.; Wang, D.H.; Tan, L.-S.; Baek, J.-B. In-Situ Grafting of Hyperbranched Poly(ether ketone)s onto Multiwalled Carbon Nanotubes via the A3 + B2 Approach. Macromolecules 2007, 40, 4474–4480. [Google Scholar] [CrossRef]
- Masukawa, S.; Kikkawa, T.; Fujimori, A.; Oishi, Y.; Shibasaki, Y. Synthesis of a A2B3-type Hyperbranched Copolymers Based on a 3-Armed Unimolecular 4-N-Methylbenzamide Pentamer and Poly(propylene oxide). Chem. Lett. 2015, 44, 536–538. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Hedstrand, D.M.; Ferritto, M.S. Comb-burst dendrimer topology: New macromolecular architecture derived from dendritic grafting. Macromolecules 1991, 24, 1435–1438. [Google Scholar] [CrossRef]
- Nguyen, C.; Hawker, C.J.; Miller, R.D.; Huang, E.; Hedrick, J.L.; Gauderon, R.; Hilborn, J.G. Hyperbranched Polyesters as Nanoporosity Templating Agents for Organosilicates. Macromolecules 2000, 33, 4281–4284. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-Assembly of Hyperbranched Polymers and Its Biomedical Applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef] [PubMed]
- Mourey, T.H.; Turner, S.R.; Rubinstein, M.; Frechet, J.M.J.; Hawker, C.J.; Wooley, K.L. Unique behavior of dendritic macromolecules: Intrinsic viscosity of polyether dendrimers. Macromolecules 1992, 25, 2401–2406. [Google Scholar] [CrossRef]
- Ohta, Y.; Sakurai, K.; Matsuda, J.; Yokozawa, T. Chain-growth condensation polymerization of 5-aminoisophthalic acid triethylene glycol ester to afford well-defined, water-soluble, thermoresponsive hyperbranched polyamides. Polymer 2016, 101, 305–310. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J. Hyperbranched polymers from A2 + B3 strategy: Recent advances in description and control of fine topology. Polym. Chem. 2016, 7, 3643–3663. [Google Scholar] [CrossRef]
- Zigmond, J.S.; Pavia-Sanders, A.; Russell, J.D.; Wooley, K.L.; Percec, V. Dynamic Anti-Icing Coatings: Complex, Amphiphilic Hyperbranched Fluoropolymer Poly(ethylene glycol) Cross-Linked Networks with an Integrated Liquid Crystalline Comonomer. Chem. Mater. 2016, 28, 5471–5479. [Google Scholar] [CrossRef]
- Zigmond, J.S.; Letteri, R.A.; Wooley, K.L.; Percec, V. Amphiphilic Cross-Linked Liquid Crystalline Fluoropolymer-Poly(ethylene glycol) Coatings for Application in Challenging Conditions: Comparative Study between Different Liquid Crystalline Comonomers and Polymer Architectures. ACS Appl. Energy Mater. 2016, 8, 33386–33393. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W.; Chen, B.; Zhuang, L.-H.; Yun, K.; Guo, J.-R.; Wang, Y.; Xu, B. Dyeing performances of ramie fabrics modified with an amino-terminated aliphatic hyperbranched polymer. Cellulose 2015, 22, 1401–1404. [Google Scholar] [CrossRef]
- Kim, Y.H.; Beckerbauer, R. Role of End Groups on the Glass Transition of Hyperbranched Polyphenylene and Triphenylbenzene Derivatives. Macromolecules 1994, 27, 1968–1971. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Wang, G.; Wu, X.; Yang, K.; Li, S.; Jiang, P. Hyperbranched-polymer functionalization of graphene sheets for enhanced mechanical and dielectric properties of polyurethane composites. J. Mater. Chem. 2012, 14, 7010–7019. [Google Scholar] [CrossRef]
- Lei, X.; Chen, Y.; Qiao, M.; Tian, L.; Zhang, Q.; Romagnoli, B. Hyperbranched polysiloxane (HBPSi)-based polyimide films with ultralow dielectric permittivity, desirable mechanical and thermal properties. J. Mater. Chem. C 2016, 11, 2134–2146. [Google Scholar] [CrossRef]
- Gadwal, I.; Binder, S.; Stuparu, M.C.; Khan, A. Dual-Reactive Hyperbranched Polymer Synthesis through Proton Transfer Polymerization of Thiol and Epoxide Groups. Macromolecules 2014, 47, 5070–5080. [Google Scholar] [CrossRef]
- Hölter, D.; Burgath, A.; Frey, H. Degree of branching in hyperbranched polymers. Acta Polym. 1997, 48, 30–35. [Google Scholar] [CrossRef]
- Kambouris, P.; Hawker, C.J. A versatile new method for structure determination in hyperbranched macromolecules. J. Chem. Soc. Perkin Trans. 1 1993, 22, 2717–2721. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Yan, D. Influence of branching architecture on polymer properties. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1277–1286. [Google Scholar] [CrossRef]
- Spears, B.R.; Waksal, J.; mcQuade, C.; Lanier, L.; Harth, E. Controlled branching of polyglycidol and formation of protein–glycidol bioconjugates via a graft-from approach with “PEG-like” arms. Chem. Commun. 2013, 49, 2394–2396. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Lin, Q.; Mourey, T.H.; Long, T.E. Tailoring the Degree of Branching: Preparation of Poly(ether ester)s via Copolymerization of Poly(ethylene glycol) Oligomers (A2) and 1,3,5-Benzenetricarbonyl Trichloride (B3). Macromolecules 2005, 38, 3246–3254. [Google Scholar] [CrossRef]
- Unal, S.; Oguz, C.; Yilgor, E.; Gallivan, M.; Long, T.E.; Yilgor, I. Understanding the structure development in hyperbranched polymers prepared by oligomeric A2 + B3 approach: Comparison of experimental results and simulations. Polymer 2005, 46, 4533–4543. [Google Scholar] [CrossRef]
- Schubert, C.; Schömer, M.; Steube, M.; Decker, S.; Friedrich, C.; Frey, H. Systematic Variation of the Degree of Branching (DB) of Polyglycerol via Oxyanionic Copolymerization of Glycidol with a Protected Glycidyl Ether and Its Impact on Rheological Properties. Macromol. Chem. Phys. 2018, 219, 1700376. [Google Scholar] [CrossRef]
- Segawa, Y.; Higashihara, T.; Ueda, M. Synthesis of hyperbranched polymers with controlled structure. Polym. Chem. 2013, 4, 1746–1759. [Google Scholar] [CrossRef]
- Mai, Y.; Zhou, Y.; Yan, D.; Lu, H. Effect of Reaction Temperature on Degree of Branching in Cationic Polymerization of 3-Ethyl-3-(hydroxymethyl)oxetane. Macromolecules 2003, 36, 9667–9669. [Google Scholar] [CrossRef]
- Popeney, C.S.; Lukowiak, M.C.; Böttcher, C.; Schade, B.; Welker, P.; Mangoldt, D.; Gunkel, G.; Guan, Z.; Haag, R. Tandem Coordination, Ring-Opening, Hyperbranched Polymerization for the Synthesis of Water-Soluble Core–Shell Unimolecular Transporters. ACS Macro Lett. 2012, 1, 564–567. [Google Scholar] [CrossRef]
- Shi, Y.; Graff, R.W.; Cao, X.; Wang, X.; Gao, H. Chain-Growth Click Polymerization of AB2 Monomers for the Formation of Hyperbranched Polymers with Low Polydispersities in a One-Pot Process. Angew. Chem. Int. Ed. 2015, 54, 7631–7635. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Higashihara, T.; Ueda, M. Hyperbranched Polymers with Controlled Degree of Branching from 0 to 100%. J. Am. Chem. Soc. 2010, 132, 11000–11001. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z. Recent Progress of Catalytic Polymerization for Controlling Polymer Topology. Chem. Asian J. 2010, 5, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z. Chain Walking: A New Strategy to Control Polymer Topology. Science 1999, 283, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, X.; Yan, D.; Chen, Y.; Chen, Q.; Yao, Y. Controlling Polymer Architecture through Host-Guest Interactions. Angew. Chem. Int. Ed. 2006, 45, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Vignolle, J.; Vincent, J.-M.; Robert, F.; Landais, Y.; Cramail, H.; Taton, D. One-Pot Synthesis and PEGylation of Hyperbranched Polyacetals with a Degree of Branching of 100%. Macromolecules 2014, 47, 1532–1542. [Google Scholar] [CrossRef]
- Radke, W.; Litvinenko, G.; Müller, A.H.E. Effect of Core-Forming Molecules on Molecular Weight Distribution and Degree of Branching in the Synthesis of Hyperbranched Polymers. Macromolecules 1998, 31, 239–248. [Google Scholar] [CrossRef]
- Ishida, Y.; Sun, A.C.F.; Jikei, M.; Kakimoto, M.-A. Synthesis of Hyperbranched Aromatic Polyamides Starting from Dendrons as ABx Monomers: Effect of Monomer Multiplicity on the Degree of Branching. Macromolecules 2000, 33, 2832–2838. [Google Scholar] [CrossRef]
- Lach, C.; Frey, H. Enhancing the Degree of Branching of Hyperbranched Polymers by Postsynthetic Modification. Macromolecules 1998, 31, 2381–2383. [Google Scholar] [CrossRef]
- Huang, W.; Su, L.; Bo, Z. Hyperbranched Polymers with a Degree of Branching of 100% Prepared by Catalyst Transfer Suzuki–Miyaura Polycondensation. J. Am. Chem. Soc. 2009, 131, 10348–10349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, D. Kinetic analysis for polycondensation of AB (g) type monomers. Chem. J. Chin. Univ. Chin. 1999, 20, 1978–1981. [Google Scholar]
- Cao, X.; Shi, Y.; Wang, X.; Graff, R.W.; Gao, H. Design a Highly Reactive Trifunctional Core Molecule To Obtain Hyperbranched Polymers with over a Million Molecular Weight in One-Pot Click Polymerization. Macromolecules 2016, 49, 760–766. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Chuang, T.-H.; Chang, J.-S.; Guo, W.; Su, W.-F. Effect of Feed Rate on Structure of Hyperbranched Polymers Formed by Self-Condensing Vinyl Polymerization in Semibatch Reactor. Macromolecules 2005, 38, 8252–8257. [Google Scholar] [CrossRef]
- Cheng, K.-C. Effect of feed rate on structure of hyperbranched polymers formed by stepwise addition of AB2 monomers into multifunctional cores. Polymer 2003, 44, 1259–1266. [Google Scholar] [CrossRef]
- Möck, A.; Burgath, A.; Hanselmann, R.; Frey, H. Synthesis of Hyperbranched Aromatic Homo- and Copolyesters via the Slow Monomer Addition Method. Macromolecules 2001, 34, 7692–7698. [Google Scholar] [CrossRef]
- Zhou, Z.; Jia, Z.; Yan, D. Effect of slow monomer addition on molecular parameters of hyperbranched polymers synthesized in the presence of multifunctional core molecules. Sci. China Chem. 2010, 53, 891–897. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Lai, W.-J. Effect of feed rate of end-capping molecules on structure of hyperbranched polymers formed from monomers A2 and B4 in semibatch process. Eur. Polym. J. 2017, 89, 339–348. [Google Scholar] [CrossRef]
- Tobita, H. Markovian Approach to Self-Condensing Vinyl Polymerization: Distributions of Molecular Weights, Degrees of Branching, and Molecular Dimensions. Macromol. Theory Simul. 2014, 24, 117–132. [Google Scholar] [CrossRef]
- Litvinenko, G.I.; Müller, A.H.E. Molecular Weight Averages and Degree of Branching in Self-Condensing Vinyl Copolymerization in the Presence of Multifunctional Initiators. Macromolecules 2002, 35, 4577–4583. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D.; Frey, H. Promising Dendritic Materials: An Introduction to Hyperbranched Polymers. In Hyperbranched Polymers: Synthesis, Properties, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–26. [Google Scholar]
- Li, Z.; Li, Q.; Qin, J. Some new design strategies for second-order nonlinear optical polymers and dendrimers. Polym. Chem. 2011, 2, 2723–2740. [Google Scholar] [CrossRef]
- Wu, W.; Huang, L.; Xiao, L.; Huang, Q.; Tang, R.; Ye, C.; Qin, J.; Li, Z. New second-order nonlinear optical (NLO) hyperbranched polymers containing isolation chromophore moieties derived from one-pot “A2 + B4” approach via Suzuki coupling reaction. RSC Adv. 2012, 2, 6520–6526. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Z.A.; Tan, Y.; Li, Z.; Li, Q.; Zeng, Q.; Ye, C.; Qin, J. New hyperbranched polymers containing second-order nonlinear optical chromophores: Synthesis and nonlinear optical characterization. Polymer 2006, 47, 7881–7888. [Google Scholar] [CrossRef]
- Bai, Y.; Song, N.; Gao, J.P.; Sun, X.; Wang, X.; Yu, G.; Wang, Z.Y. A New Approach to Highly Electrooptically Active Materials Using Cross-Linkable, Hyperbranched Chromophore-Containing Oligomers as a Macromolecular Dopant. J. Am. Chem. Soc. 2005, 127, 2060–2061. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qin, A.; Lam, J.W.Y.; Dong, Y.; Dong, Y.; Ye, C.; Williams, I.D.; Tang, B.Z. Facile Synthesis, Large Optical Nonlinearity, and Excellent Thermal Stability of Hyperbranched Poly(aryleneethynylene)s Containing Azobenzene Chromophores. Macromolecules 2006, 39, 1436–1442. [Google Scholar] [CrossRef]
- Scarpaci, A.; Blart, E.; Montembault, V.R.; Fontaine, L.; Rodriguez, V.; Odobel, F. Synthesis and Nonlinear Optical Properties of a Peripherally Functionalized Hyperbranched Polymer by DR1 Chromophores. ACS Appl. Mater. Interfaces 2009, 1, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Scarpaci, A.; Blart, E.; Montembault, V.; Fontaine, L.; Rodriguez, V.; Odobel, F. A new crosslinkable system based on thermal Huisgen reaction to enhance the stability of electro-optic polymers. Chem. Commun. 2009, 14, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bo, Z. “AB2 + AB” Approach to Hyperbranched Polymers Used as Polymer Blue Light Emitting Materials. Macromolecules 2004, 37, 2013–2015. [Google Scholar] [CrossRef]
- Xin, Y.; Wen, G.-A.; Zeng, W.-J.; Zhao, L.; Zhu, X.-R.; Fan, Q.-L.; Feng, J.-C.; Wang, L.-H.; Peng, B.; Cao, Y.; et al. Hyperbranched Oxadiazole-Containing Polyfluorenes: Toward Stable Blue Light PLEDs. Macromolecules 2005, 38, 6755–6758. [Google Scholar] [CrossRef]
- Tsai, L.-R.; Chen, Y. Novel Hyperbranched Polyfluorenes Containing Electron-Transporting Aromatic Triazole as Branch Unit. Macromolecules 2007, 40, 2984–2992. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Zhou, X.-H.; Zi, H.; Pei, J. Novel Blue-Light-Emitting Truxene-Containing Hyperbranched and Zigzag Type Copolymers: Synthesis, Optical Properties, and Investigation of Thermal Spectral Stability. Macromolecules 2004, 37, 8874–8882. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, X.; Wu, J.; Jin, J.; Ba, X. Pure Blue-Light-Emitting Materials: Hyperbranched Ladder-Type Poly(p-phenylene)s Containing Truxene Units. Macromolecules 2010, 43, 731–738. [Google Scholar] [CrossRef]
- Wu, G.; Yang, Y.; He, C.; Chen, X.; Li, Y. A new triphenylamine-based hyperbranched polyfluorene with oxadiazole units on its side chains. Eur. Polym. J. 2008, 44, 4047–4053. [Google Scholar] [CrossRef]
- Li, Z.A.; Ye, S.; Liu, Y.; Yu, G.; Wu, W.; Qin, J.; Li, Z. New Hyperbranched Conjugated Polymers Containing Hexaphenylbenzene and Oxadiazole Units: Convenient Synthesis and Efficient Deep Blue Emitters for PLEDs Application. J. Phys. Chem. B 2010, 114, 9101–9108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, L.; Zhong, C.; He, R.; Yang, W.; Wu, H.; Cao, Y. Highly efficient green-emitting electrophosphorescent hyperbranched polymers using a bipolar carbazole-3,6-diyl-co-2,8-octyldibenzothiophene-S,S-dioxide-3,7-diyl unit as the branch. RSC Adv. 2012, 2, 689–696. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, L.; Gao, C. Hyperbranched polymers meet colloid nanocrystals: A promising avenue to multifunctional, robust nanohybrids. Colloid Polym. Sci. 2011, 289, 1299–1320. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, F.; Zhu, B.; Zhu, X. Hyperbranched polymers for bioimaging. RSC Adv. 2013, 3, 2071–2083. [Google Scholar] [CrossRef]
- Pérignon, N.; Marty, J.-D.; Mingotaud, A.-F.; Dumont, M.; Rico-Lattes, I.; Mingotaud, C. Hyperbranched Polymers Analogous to PAMAM Dendrimers for the Formation and Stabilization of Gold Nanoparticles. Macromolecules 2007, 40, 3034–3041. [Google Scholar] [CrossRef]
- Saliba, S.; Valverde Serrano, C.; Keilitz, J.; Kahn, M.L.; Mingotaud, C.; Haag, R.; Marty, J.-D. Hyperbranched Polymers for the Formation and Stabilization of ZnO Nanoparticles. Chem. Mater. 2010, 22, 6301–6309. [Google Scholar] [CrossRef]
- Tuchbreiter, L.; Mecking, S. Hydroformylation with Dendritic-Polymer-Stabilized Rhodium Colloids as Catalyst Precursors. Macromol. Chem. Phys. 2007, 208, 1688–1693. [Google Scholar] [CrossRef]
- Gladitz, M.; Reinemann, S.; Radusch, H.-J. Preparation of Silver Nanoparticle Dispersions via a Dendritic-Polymer Template Approach and their Use for Antibacterial Surface Treatment. Macromol. Mater. Eng. 2009, 294, 178–189. [Google Scholar] [CrossRef]
- Krämer, M.; Pérignon, N.; Haag, R.; Marty, J.-D.; Thomann, R.; Lauth-de Viguerie, N.; Mingotaud, C. Water-Soluble Dendritic Architectures with Carbohydrate Shells for the Templation and Stabilization of Catalytically Active Metal Nanoparticles. Macromolecules 2005, 38, 8308–8315. [Google Scholar] [CrossRef]
- Shen, Z.; Duan, H.; Frey, H. Water-Soluble Fluorescent Ag Nanoclusters Obtained from Multiarm Star Poly(acrylic acid) as “Molecular Hydrogel” Templates. Adv. Mater. 2007, 19, 349–352. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, C.; Hu, X.; Xu, W. General Avenue to Multifunctional Aqueous Nanocrystals Stabilized by Hyperbranched Polyglycerol. Chem. Mater. 2011, 23, 1461–1470. [Google Scholar] [CrossRef]
- Wan, D.; Fu, Q.; Huang, J. Synthesis of a thioether modified hyperbranched polyglycerol and its template effect on fabrication of CdS and CdSe nanoparticles. J. Appl. Polym. Sci. 2006, 102, 3679–3684. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, B.; Xu, Y. Preparation and stability of copper particles formed using the template of hyperbranched poly(amine-ester). Colloid Polym. Sci. 2005, 284, 102–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, J.; Shi, W. Synthesis and characterization of PbS/modified hyperbranched polyester nanocomposite hollow spheres at room temperature. Mater. Lett. 2005, 59, 686–689. [Google Scholar] [CrossRef]
- Monticelli, O.; Russo, S.; Campagna, R.; Voit, B. Preparation and characterisation of blends based on polyamide 6 and hyperbranched aramids as palladium nanoparticle supports. Polymer 2005, 46, 3597–3606. [Google Scholar] [CrossRef]
- Kakati, N.; Mahapatra, S.S.; Karak, N. Silver Nanoparticles in Polyacrylamide and Hyperbranched Polyamine Matrix. J. Macromol. Sci. Part A Pure Appl. Chem. 2008, 45, 658–663. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Karak, N. Silver nanoparticle in hyperbranched polyamine: Synthesis, characterization and antibacterial activity. Mater. Chem. Phys. 2008, 112, 1114–1119. [Google Scholar] [CrossRef]
- Richter, T.V.; Schüler, F.; Thomann, R.; Mülhaupt, R.; Ludwigs, S. Nanocomposites of Size-Tunable ZnO-Nanoparticles and Amphiphilic Hyperbranched Polymers. Macromol. Rapid Commun. 2009, 30, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Guizhe, Z.; Zhang, Q. Effects of hyperbranched poly(amido-amine)s structures on synthesis of Ag particles. J. Appl. Polym. Sci. 2007, 107, 9–13. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.; Mazurkiewicz-Pawlicka, M.; Malinowka, E. Peroxidase-like activity of gold nanoparticles stabilized by hyperbranched polyglycidol derivatives over a wide pH range. Nanotechnology 2015, 16, 495101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, Y.; Tu, C.; Wang, R.; Pang, Y.; Qiu, F.; Zhu, X.; Yan, D.; He, L.; Jin, C.; et al. Construction and Application of a pH-Sensitive Nanoreactor via a Double-Hydrophilic Multiarm Hyperbranched Polymer. Langmuir 2010, 26, 8875–8881. [Google Scholar] [CrossRef] [PubMed]
- Keilitz, J.; Radowski, M.R.; Marty, J.-D.; Haag, R.; Gauffre, F.; Mingotaud, C. Dendritic Polymers with a Core–Multishell Architecture: A Versatile Tool for the Stabilization of Nanoparticles. Chem. Mater. 2008, 20, 2423–2425. [Google Scholar] [CrossRef]
- Moisan, S.; Martinez, V.; Weisbecker, P.; Cansell, F.; Mecking, S.; Aymonier, C. General Approach for the Synthesis of Organic–Inorganic Hybrid Nanoparticles Mediated by Supercritical CO2. J. Am. Chem. Soc. 2007, 129, 10602–10606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Frey, H.; Thomann, R.; Stiriba, S.-E. Optically active amphiphilic hyperbranched polyglycerols as templates for palladium nanoparticles. Inorg. Chim. Acta 2006, 359, 1837–1844. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, C.; Hu, X.; Xu, W. One-Pot Large-Scale Synthesis of Robust Ultrafine Silica-Hybridized CdTe Quantum Dots. ACS Appl. Mater. Interfaces 2010, 2, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, C.; Xu, W. Magnetic Dendritic Materials for Highly Efficient Adsorption of Dyes and Drugs. ACS Appl. Mater. Interfaces 2010, 2, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, C.; Xu, W.; Wang, X.; Xu, Y. Enhanced Biocompatibility and Biostability of CdTe Quantum Dots by Facile Surface-Initiated Dendritic Polymerization. Biomacromolecules 2009, 10, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Du, J.; Zhou, L.; Li, X.; Zhou, Y.; Li, L.; Zang, X.; Zhang, X.; Pan, F.; Zhang, H.; et al. Size-controlled preparation of magnetic iron oxidenanocrystals within hyperbranched polymers and their magnetofection in vitro. J. Mater. Chem. 2012, 22, 355–360. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, X.; Yin, J. Responsive hybrid nanosheets of hyperbranched poly(ether amine) as a 2D-platform for metal nanoparticles. Chem. Commun. 2013, 49, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D. Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: Progress, characteristics and perspectives. Chem. Commun. 2009, 10, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Blakey, I.; Thurecht, K.J.; Fredericks, P.M. Self-Assembled Hyperbranched Polymer–Gold Nanoparticle Hybrids: Understanding the Effect of Polymer Coverage on Assembly Size and SERS Performance. Langmuir 2013, 29, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kuang, M.; Shen, Z.; Nieberle, J.; Duan, H.; Frey, H. Gold Nanoparticles Coated with a Thermosensitive Hyperbranched Polyelectrolyte: Towards Smart Temperature and pH Nanosensors. Angew. Chem. Int. Ed. 2008, 47, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Huang, W.; Zhu, X.; Zhou, Y.; Yan, D. Biocompatible or biodegradable hyperbranched polymers: From self-assembly to cytomimetic applications. Chem. Soc. Rev. 2012, 41, 5986–5997. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, H.; Su, Y.; Qiu, F.; Zhu, L.; Huan, X.; Zhu, B.; Yan, D.; Guo, F.; Zhu, X. Supramolecular amphiphilic multiarm hyperbranched copolymer: Synthesis, self-assembly and drug delivery applications. Polym. Chem. 2013, 4, 85–94. [Google Scholar] [CrossRef]
- Hartlieb, M.; Floyd, T.; Cook, A.B.; Sanchez-Cano, C.; Catrouillet, S.; Burns, J.A.; Perrier, S. Well-defined hyperstar copolymers based on a thiol–yne hyperbranched core and a poly(2-oxazoline) shell for biomedical applications. Polym. Chem. 2017, 13, 2014–2054. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D. Real-Time Membrane Fusion of Giant Polymer Vesicles. J. Am. Chem. Soc. 2005, 127, 10468–10469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D. Real-Time Membrane Fission of Giant Polymer Vesicles. Angew. Chem. Int. Ed. 2005, 44, 3223–3226. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature’s building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Wu, J.; Gao, C.; Xu, Y. Synthesis and Evaluation of Phenylalanine-Modified Hyperbranched Poly(amido amine)s as Promising Gene Carriers. Biomacromolecules 2010, 11, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, X.; Pan, D.; Mao, H.-Q. Charge Density and Molecular Weight of Polyphosphoramidate Gene Carrier Are Key Parameters Influencing Its DNA Compaction Ability and Transfection Efficiency. Biomacromolecules 2010, 11, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Newland, B.; Tai, H.; Zheng, Y.; Velasco, D.; Di Luca, A.; Howdle, S.M.; Alexander, C.; Wang, W.; Pandit, A. A highly effective gene delivery vector—Hyperbranched poly(2-(dimethylamino)ethyl methacrylate) from in situ deactivation enhanced ATRP. Chem. Commun. 2010, 46, 4698–4700. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, J.; Zhou, L.; Jin, C.; Tu, C.; Zhu, B.; Wu, F.; Zhu, Q.; Zhu, X.; Yan, D. Hyperbranched glycoconjugated polymer from natural small molecule kanamycin as a safe and efficient gene vector. Polym. Chem. 2011, 2, 2674–2682. [Google Scholar] [CrossRef]
- Tu, C.; Li, N.; Zhu, L.; Zhou, L.; Su, Y.; Li, P.; Zhu, X. Cationic long-chain hyperbranched poly(ethylene glycol)s with low charge density for gene delivery. Polym. Chem. 2013, 4, 393–401. [Google Scholar] [CrossRef]
- Yu, S.; Chen, J.; Dong, R.; Su, Y.; Ji, B.; Zhou, Y.; Zhu, X.; Yan, D. Enhanced gene transfection efficiency of PDMAEMA by incorporating hydrophobic hyperbranched polymer cores: Effect of degree of branching. Polym. Chem. 2012, 3, 3324–3329. [Google Scholar] [CrossRef]
- Wang, G.; Yin, H.; Yin Ng, J.C.; Cai, L.; Li, J.; Tang, B.Z.; Liu, B. Polyethyleneimine-grafted hyperbranched conjugated polyelectrolytes: Synthesis and imaging of gene delivery. Polym. Chem. 2013, 4, 5297–5304. [Google Scholar] [CrossRef]
- Siegers, C.; Biesalski, M.; Haag, R. Self-Assembled Monolayers of Dendritic Polyglycerol Derivatives on Gold That Resist the Adsorption of Proteins. Chem. Eur. J. 2004, 10, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tan, Z.; Li, N.; Wang, R.; He, L.; Shi, Y.; Jiang, L.; Li, P.; Zhu, X. Highly Efficient Intracellular Drug Delivery with a Negatively Charged Hyperbranched Polysulfonamine. Macromol. Biosci. 2011, 11, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Xu, J.; Li, X.; Zhong, L.; Li, J.; Li, J.; Nan, F. Design and Synthesis of Matrix Metalloprotease Photoaffinity Trimodular Probes. Chin. J. Chem. 2009, 27, 825–833. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, D.; Zhu, Q.; Zhu, L.; Tong, G.; Lu, Y.; Yan, D.; Zhu, X. Real-Time Monitoring of Anticancer Drug Release with Highly Fluorescent Star-Conjugated Copolymer as a Drug Carrier. Biomacromolecules 2014, 15, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Sohn, G.-J.; Choi, H.-J.; Jeon, I.-Y.; Chang, D.W.; Dai, L.; Baek, J.-B. Water-Dispersible, Sulfonated Hyperbranched Poly(ether-ketone) Grafted Multiwalled Carbon Nanotubes as Oxygen Reduction Catalysts. ACS Nano 2012, 6, 6345–6355. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F. Nanoengineering of Particle Surfaces. Adv. Mater. 2001, 13, 11–22. [Google Scholar] [CrossRef]
- Hood, M.; Mari, M.; Muñoz-Espí, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Lee, H.-J.; Choi, Y.S.; Tan, L.-S.; Baek, J.-B. Semimetallic Transport in Nanocomposites Derived from Grafting of Linear and Hyperbranched Poly(phenylene sulfide)s onto the Surface of Functionalized Multi-Walled Carbon Nanotubes. Macromolecules 2008, 41, 7423–7432. [Google Scholar] [CrossRef]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Roldán, R.; Chirolli, L.; Prada, E.; Silva-Guillén, J.A.; San-Jose, P.; Guinea, F. Theory of 2D crystals: Graphene and beyond. Chem. Soc. Rev. 2017, 15, 4387–4399. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Choi, H.-J.; Bae, S.-Y.; Chang, D.W.; Baek, J.-B. Wedging graphite into graphene and graphene-like platelets by dendritic macromolecules. J. Mater. Chem. 2011, 21, 7820–7826. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Gotou, T.; Ohba, M. Thin-film particles of graphite oxide. Preliminary studies for internal micro fabrication of single particle and carbonaceous electronic circuits. Carbon 2005, 43, 503–510. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Z.; Liu, Z.; Gao, C. Liquid crystal self-templating approach to ultrastrong and tough biomimic composites. Sci. Rep. 2013, 3, 2374. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, C. Graphene in Macroscopic Order: Liquid Crystals and Wet-Spun Fibers. Acc. Chem. Res. 2014, 47, 1267–1276. [Google Scholar] [CrossRef] [PubMed]










| Linear | Hyperbranched | Dendrimer | |
|---|---|---|---|
| Structure | 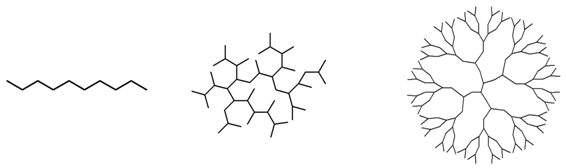 | ||
| Topology | 1D, linear | 3D, irregular | 3D, regular |
| Synthesis | One-step, facile | One-step, relatively facile | Multi-step, laborious |
| Purification | Precipitation | Precipitation or classification | Chromatography |
| Scaling-up | Already, easy | Already, easy | Difficult |
| MW 1 | Discrepant | Discrepant | Identical |
| PDI 2 | >1.1 | >1.1 | 1.0 (<1.05) |
| DB 3 | 0 | 0.4–0.6 | 1.0 |
| Entanglement | Strong | Weak | Very weak or none |
| Viscosity | High | Low | Very low |
| Solubility | Low | High | High |
| Functional group | At two ends | At linear and terminal units | On periphery (terminal units) |
| Reactivity | Low | High | High |
| Strength | High | Low | Very low |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, I.-Y.; Noh, H.-J.; Baek, J.-B. Hyperbranched Macromolecules: From Synthesis to Applications. Molecules 2018, 23, 657. https://doi.org/10.3390/molecules23030657
Jeon I-Y, Noh H-J, Baek J-B. Hyperbranched Macromolecules: From Synthesis to Applications. Molecules. 2018; 23(3):657. https://doi.org/10.3390/molecules23030657
Chicago/Turabian StyleJeon, In-Yup, Hyuk-Jun Noh, and Jong-Beom Baek. 2018. "Hyperbranched Macromolecules: From Synthesis to Applications" Molecules 23, no. 3: 657. https://doi.org/10.3390/molecules23030657




