Antiviral Activity of Novel Quinoline Derivatives against Dengue Virus Serotype 2
Abstract
:1. Introduction
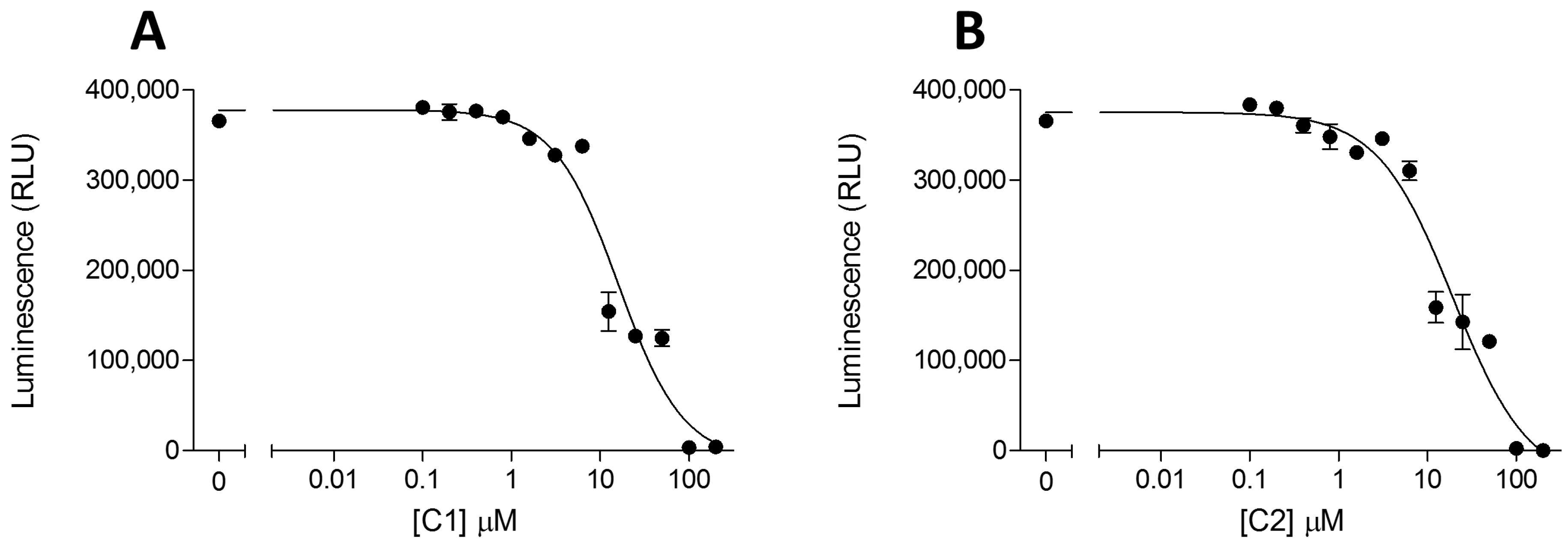
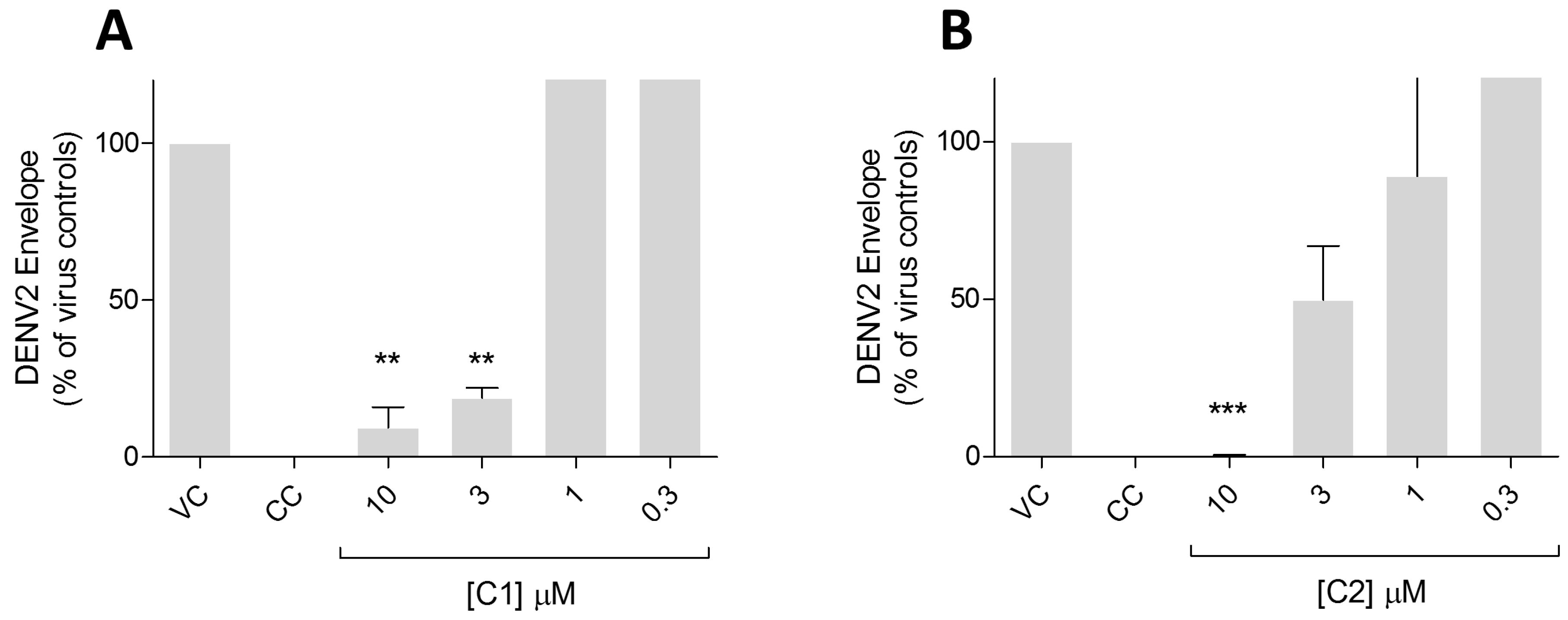
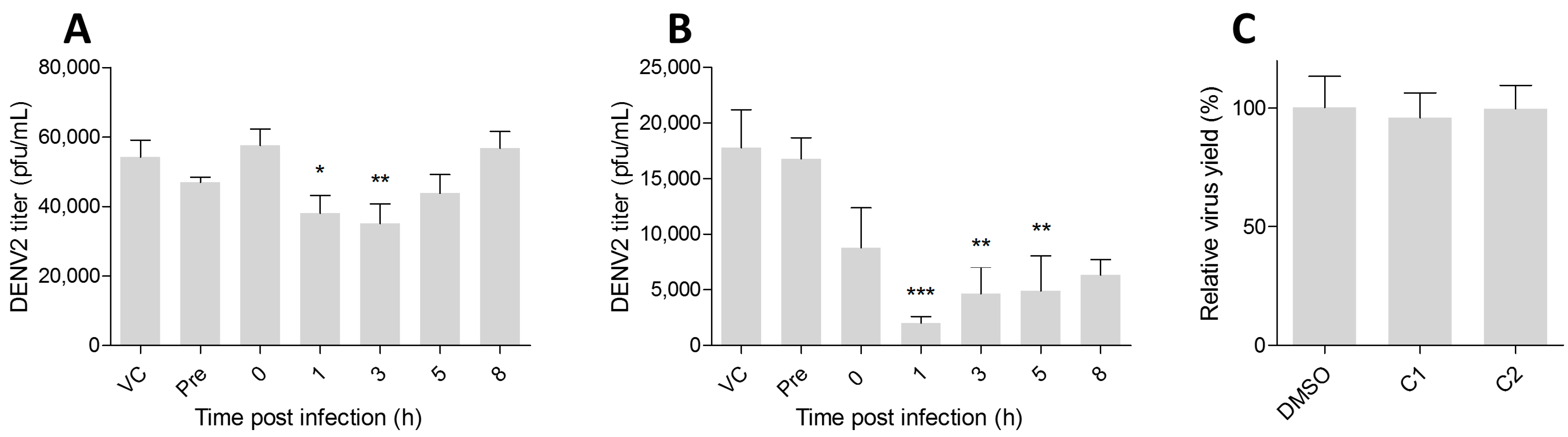
2. Results
3. Discussion
4. Materials and Methods
4.1. Cells, Viruses and Compounds
4.2. Synthetic Procedures
4.3. Primary Screening Antiviral Activity Assay
4.4. Confirmatory Virus Yield Reduction Assay
4.5. Cytotoxicity Assay
4.6. Western Blot Assay
4.7. Time-of-Addition Analysis
4.8. Virucidal Activity Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, C.P.; Farrar, J.J.; van Nguyen, V.C.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sangkawibha, N.; Rojanasuphot, S.; Ahandrik, S.; Viriyapongse, S.; Jatanasen, S.; Salitul, V.; Phanthumachinda, B.; Halstead, S.B. Risk factors in dengue shock syndrome: A prospective epidemiologic study in Rayong, Thailand: I. The 1980 outbreak. Am. J. Epidemiol. 1984, 120, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Rothman, A.L. Trials and Tribulations on the Path to Developing a Dengue Vaccine. Am. J. Prev. Med. 2015, 49, S334–S344. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Licensed Dengue Vaccine: Public Health Conundrum and Scientific Challenge. Am. J. Trop. Med. Hyg. 2016, 95, 741–745. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and Special Programme for Research and Training in Tropical Diseases. Handbook for Clinical Management of Dengue. WHO 2014. Available online: http://www.who.int/denguecontrol/9789241504713/en/ (accessed on 26 January 2018).
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-H.; Lee, K.K.-H.; Gambari, R.; Kok, S.H.-L.; Kok, T.-W.; Chan, A.S.-C.; Bian, Z.-X.; Wong, W.-Y.; Wong, R.S.-M.; Lau, F.-Y.; et al. Anti-tumour and pharmacokinetics study of 2-Formyl-8-hydroxy-quinolinium chloride as Galipea longiflora alkaloid analogue. Phytomedicine 2014, 21, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-H.; Gambari, R.; Lee, K.K.-H.; Chen, Y.-X.; Kok, S.H.-L.; Wong, R.S.-M.; Lau, F.-Y.; Cheng, C.-H.; Wong, W.-Y.; Bian, Z.-X.; et al. Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2014, 24, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, S.; Tran, H.G.; Desmet, T.; D’hooghe, M. Evaluation of (4-aminobutyloxy)quinolines as a novel class of antifungal agents. Bioorg. Med. Chem. Lett. 2013, 23, 4641–4643. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.; Vargas Mendez, L.; Milena Leal, S.; Mora Cruz, U.; Andres Coronado, C.; Melendez Gomez, C.; Romero Bohorquez, A.; Escobar Rivero, P. Target-Oriented Synthesis of Antiparasitic 2-Hetaryl Substituted Quinolines Based on Imino Diels-Alder Reactions. Lett. Drug Des. Discov. 2007, 4, 293–296. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Colson, P.; Raoult, D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 2007, 30, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, L.; Wang, B.; Miao, K.; Xiang, K.; Feng, S.; Gao, L.; Shen, H.C.; Yun, H. Discovery of Piperazinylquinoline Derivatives as Novel Respiratory Syncytial Virus Fusion Inhibitors. ACS Med. Chem. Lett. 2016, 7, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Talamas, F.X.; Abbot, S.C.; Anand, S.; Brameld, K.A.; Carter, D.S.; Chen, J.; Davis, D.; de Vicente, J.; Fung, A.D.; Gong, L.; et al. Discovery of N-[4-[6-tert-Butyl-5-methoxy-8-(6-methoxy-2-oxo-1-H-pyridin-3-yl)-3-quinolyl]phenyl]methanesulfonamide (RG7109), a Potent Inhibitor of the Hepatitis C Virus NS5B Polymerase. J. Med. Chem. 2014, 57, 1914–1931. [Google Scholar] [CrossRef] [PubMed]
- Goodell, J.R.; Puig-Basagoiti, F.; Forshey, B.M.; Shi, P.; Ferguson, D.M. Identification of Compounds with Anti-West Nile Virus Activity. J. Med. Chem. 2006, 49, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Ezgimen, M.; Lai, H.; Mueller, N.H.; Lee, K.; Cuny, G.; Ostrov, D.A.; Padmanabhan, R. Characterization of the 8-hydroxyquinoline scaffold for inhibitors of West Nile virus serine protease. Antivir. Res. 2012, 94, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Lien, J.-C.; Chen, C.-J.; Liu, Y.-C.; Wang, C.-Y.; Ping, C.-F.; Lin, Y.-F.; Huang, A.-C.; Lin, C.-W. Antiviral Activity of a Novel Compound CW-33 against Japanese Encephalitis Virus through Inhibiting Intracellular Calcium Overload. Int. J. Mol. Sci. 2016, 17, 1386. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Lima, G.; Moraes, A.M.; da Araújo, A.S.; da Silva, E.T.; de Freitas, C.S.; Vieira, Y.R.; Marttorelli, A.; Neto, J.C.; Bozza, P.T.; de Souza, M.V.N.; et al. 2,8-Bis(trifluoromethyl)quinoline analogs show improved anti-Zika virus activity, compared to mefloquine. Eur. J. Med. Chem. 2017, 127, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Sridhar Prasad, G.; Padmanabhan, R. Characterization of 8-hydroxyquinoline derivatives containing aminobenzothiazole as inhibitors of dengue virus type 2 protease in vitro. Antivir. Res. 2013, 97, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Larionov, O.V.; Stephens, D.; Mfuh, A.M.; Arman, H.D.; Naumova, A.S.; Chavez, G.; Skenderi, B. Insights into the mechanistic and synthetic aspects of the Mo/P-catalyzed oxidation of N-heterocycles. Org. Biomol. Chem. 2014, 12, 3026–3036. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.E.; Larionov, O.V. Recent Advances in the C-H-Functionalization of the Distal Positions in Pyridines and Quinolines. Tetrahedron 2015, 71, 8683–8716. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.E.; Larionov, O.V. Transition Metal-Catalyzed C-H Functionalization of Heterocyclic N-Oxides. Top. Heterocycl. Chem. 2017, 56–84. [Google Scholar] [CrossRef]
- Stephens, D.E.; Nguyen, V.T.; Chhetri, B.; Clark, E.R.; Arman, H.D.; Larionov, O.V. Organocatalytic synthesis of methylene-bridged N-heterobiaryls. Org. Lett. 2016, 18, 5808–5811. [Google Scholar] [CrossRef] [PubMed]
- Larionov, O.V.; Stephens, D.; Mfuh, A.; Chavez, G. Direct, catalytic, and regioselective synthesis of 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles from N-oxides. Org. Lett. 2014, 16, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Khadem, T.M.; Brown, J. A review of tuberculosis: Focus on bedaquiline. Am. J. Health. Syst. Pharm. 2013, 70, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.H.; Pattabiraman, N.; Ansarah-Sobrinho, C.; Viswanathan, P.; Pierson, T.C.; Padmanabhan, R. Identification and Biochemical Characterization of Small-Molecule Inhibitors of West Nile Virus Serine Protease by a High-Throughput Screen. Antimicrob. Agents Chemother. 2008, 52, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Boonyasuppayakorn, S.; Reichert, E.D.; Manzano, M.; Nagarajan, K.; Padmanabhan, R. Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antivir. Res. 2014, 106, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Randolph, V.B.; Winkler, G.; Stollar, V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 1990, 174, 450–458. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet. Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Savarino, A.; Di Trani, L.; Donatelli, I.; Cauda, R.; Cassone, A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006, 6, 67–69. [Google Scholar] [CrossRef]
- Di Trani, L.; Savarino, A.; Campitelli, L.; Norelli, S.; Puzelli, S.; D’Ostilio, D.; Vignolo, E.; Donatelli, I.; Cassone, A. Different pH requirements are associated with divergent inhibitory effects of chloroquine on human and avian influenza A viruses. Virol. J. 2007, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Martinson, J.A.; Montoya, C.J.; Usuga, X.; Ronquillo, R.; Landay, A.L.; Desai, S.N. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: Implication for T-cell activation. Antimicrob. Agents Chemother. 2010, 54, 871–881. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Chlorquinaldol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6301 (accessed on 25 August 2017).
- Rohde, W.; Mikelens, P.; Jackson, J.; Blackman, J.; Whitcher, J.; Levinson, W. Hydroxyquinolines inhibit ribonucleic acid-dependent deoxyribonucleic acid polymerase and inactivate Rous sarcoma virus and herpes simplex virus. Antimicrob. Agents Chemother. 1976, 10, 234–240. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors upon request. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Guardia, C.; Stephens, D.E.; Dang, H.T.; Quijada, M.; Larionov, O.V.; Lleonart, R. Antiviral Activity of Novel Quinoline Derivatives against Dengue Virus Serotype 2. Molecules 2018, 23, 672. https://doi.org/10.3390/molecules23030672
De la Guardia C, Stephens DE, Dang HT, Quijada M, Larionov OV, Lleonart R. Antiviral Activity of Novel Quinoline Derivatives against Dengue Virus Serotype 2. Molecules. 2018; 23(3):672. https://doi.org/10.3390/molecules23030672
Chicago/Turabian StyleDe la Guardia, Carolina, David E. Stephens, Hang T. Dang, Mario Quijada, Oleg V. Larionov, and Ricardo Lleonart. 2018. "Antiviral Activity of Novel Quinoline Derivatives against Dengue Virus Serotype 2" Molecules 23, no. 3: 672. https://doi.org/10.3390/molecules23030672
APA StyleDe la Guardia, C., Stephens, D. E., Dang, H. T., Quijada, M., Larionov, O. V., & Lleonart, R. (2018). Antiviral Activity of Novel Quinoline Derivatives against Dengue Virus Serotype 2. Molecules, 23(3), 672. https://doi.org/10.3390/molecules23030672





