Association of Melatonin Production with Seasonal Changes, Low Temperature, and Immuno-Responses in Hamsters
Abstract
:1. Introduction
2. Results
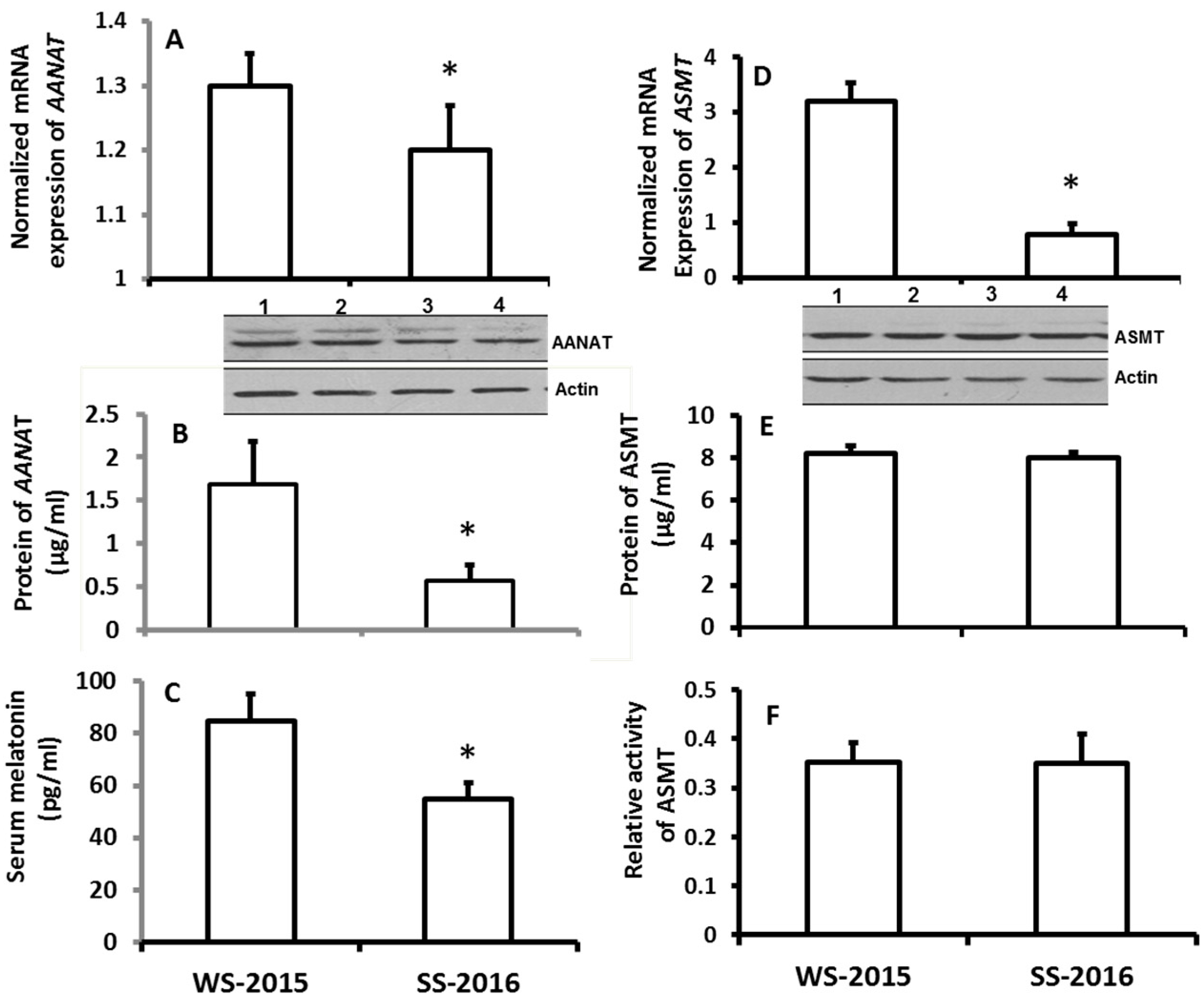
2.1. Effects of Seasonal Changes on the Gene Expression of AANAT and SAMT in Pineal Glands of Hamsters As Well As on Their Serum Melatonin Levels
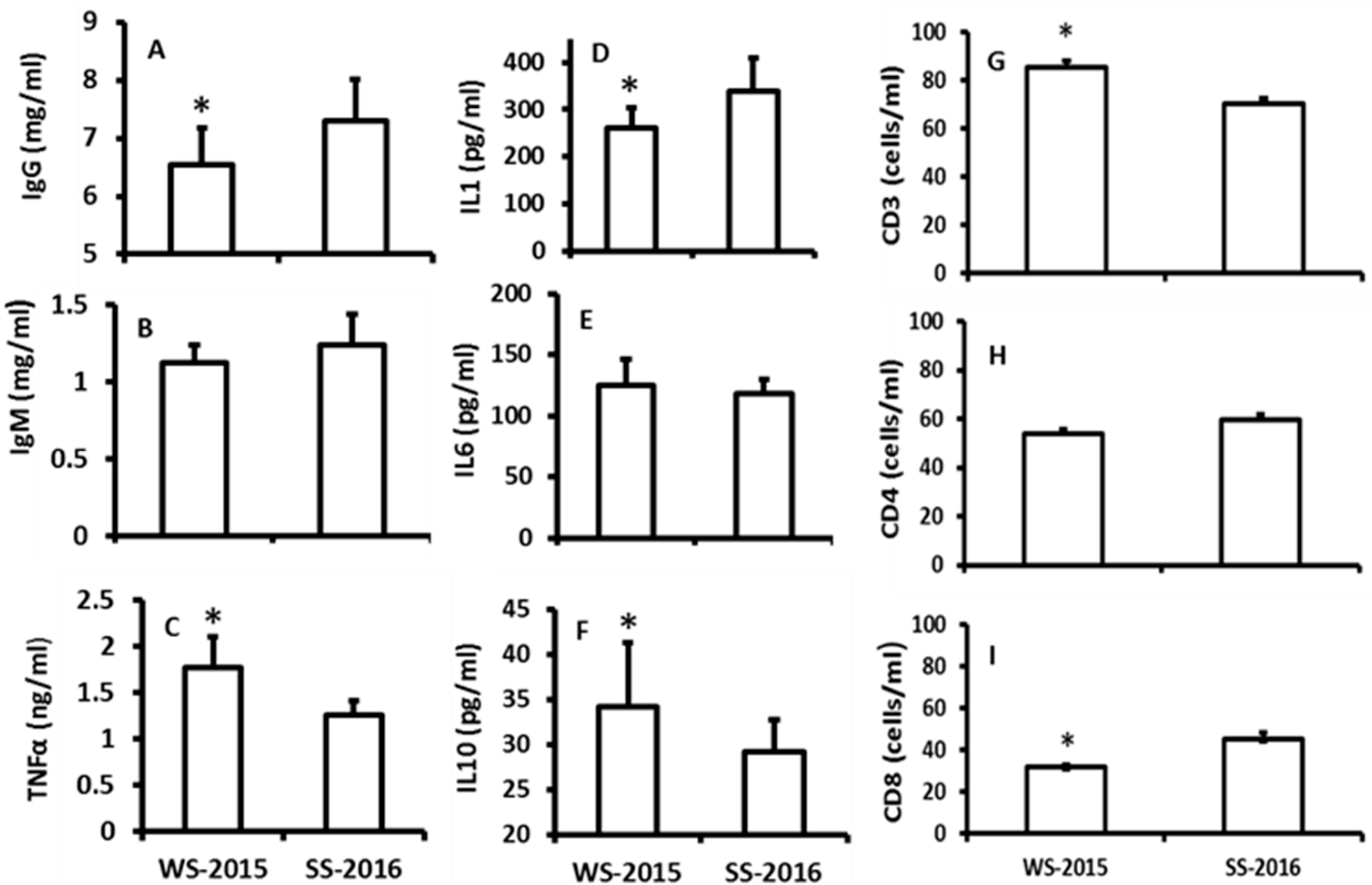
2.2. The Influence of the Seasonal Changes on the Indexes of Immuno-Responses
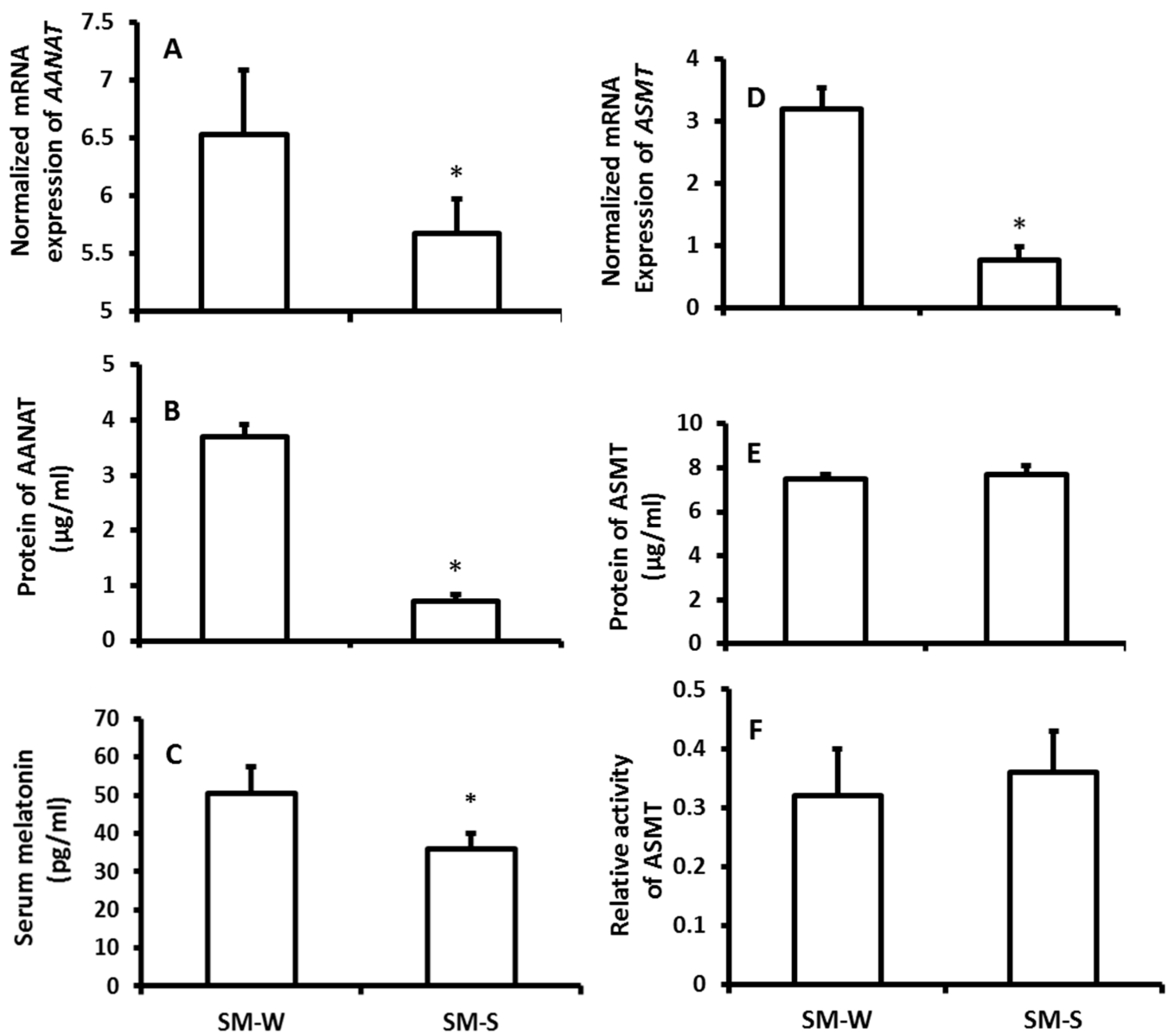
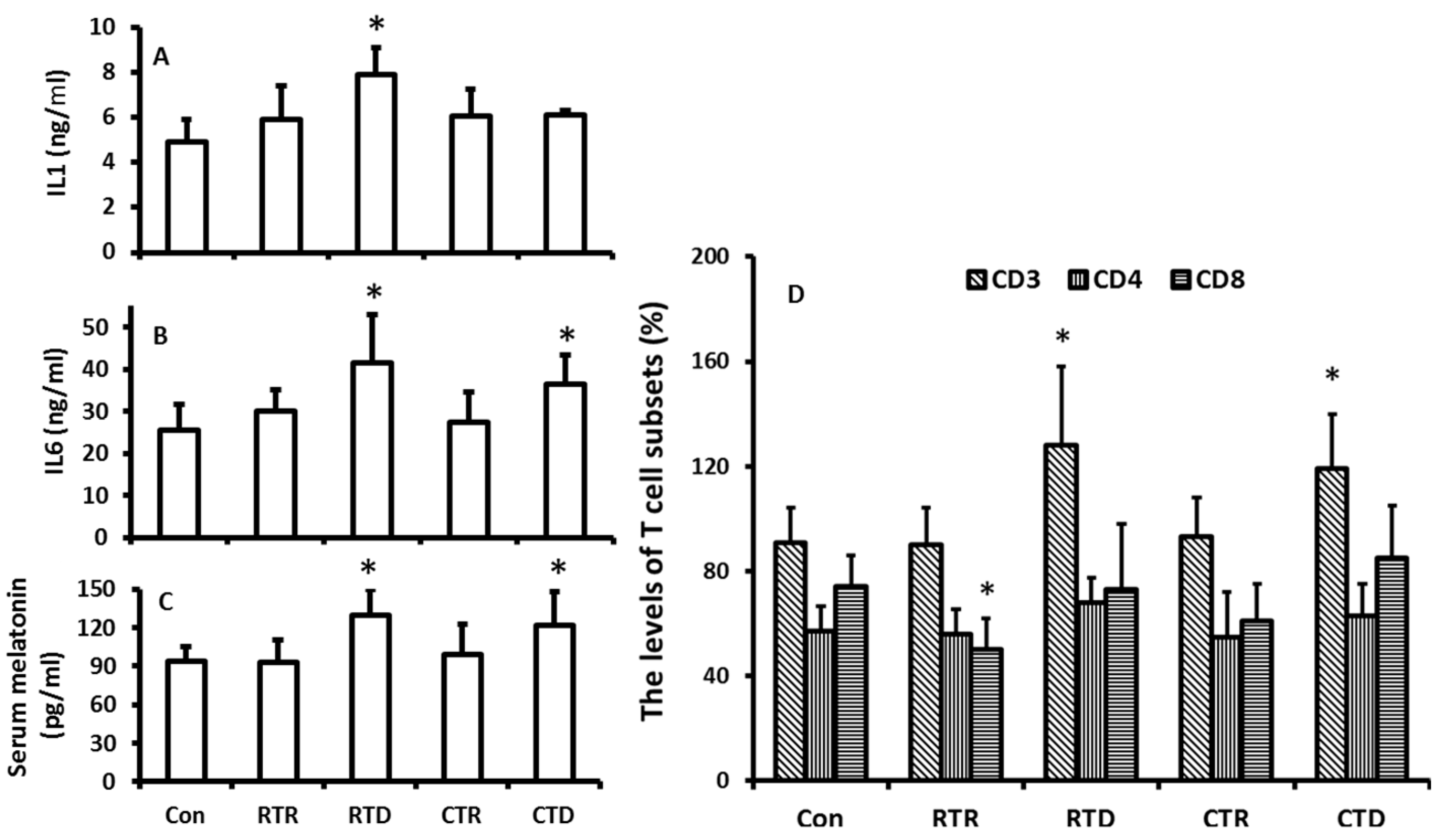
2.3. The Effects of Temperature Changes on Melatonin Production and the Indexes of Immuno-Responses
3. Discussions
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Animal Studies
4.3.1. Study of Natural Seasonal Changes on the Expression of Melatonin Synthetic Genes Including AANAT and ASMT in the Pineal Gland and Circulating Melatonin Production
4.3.2. Study of Simulated Summer and Winter Climate Changes on the Expression of Melatonin-Synthetic Genes of the Pineal Gland and Melatonin Production
4.3.3. Study of Extreme temperature Alterations on Melatonin Production and Immune-Response of Hamsters
4.4. Methods
4.4.1. Melatonin Assay
4.4.2. Measurement of mRNA Expression of AANAT and ASMT in the Pineal Glands
4.4.3. Analysis of Protein Levels of AANAT and ASMT of the Pineal Glans
4.4.4. Measurement of the Activity of ASMT
4.4.5. Measurements of TNF-α, IL-1, IL-6, IgG, and IgM
4.4.6. Detection of T Lymphocyte Subsets (CD3, CD4, and CD8)
4.5. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tan, D.X.; Manchester, L.C.; Reiter, R.J. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med. Hypotheses 2016, 86, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tosches, M.A.; Bucher, D.; Vopalensky, P.; Arendt, D. Melatonin Signaling Controls Circadian Swimming Behavior in Marine Zooplankton. Cell 2014, 159, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Singh, A.K.; Haldar, C. Seasonal modulation of immunity by melatonin and gonadal steroids in a short day breeder goat Capra hircus. Theriogenology 2014, 82, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, G.H.; Omar, H.M.; Hassanein, A.-F.; Cuzzocrea, S.; Reiter, R.J. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic. Biol. Med. 2002, 32, 319–332. [Google Scholar] [CrossRef]
- Wongsena, W.; Charoensuk, L.; Dangtakot, R.; Pinlaor, P.; Intuyod, K.; Pinlaor, S. Melatonin suppresses eosinophils and Th17 cells in hamsters treated with a combination of human liver fluke infection and a chemical carcinogen. Pharmacol. Rep. 2017, 70, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Korkmaz, A.; Reiter, R.J.; Manchester, L.C. Ebola virus disease: Potential use of melatonin as a treatment. J. Pineal Res. 2014, 57, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hu, W.; Wang, Q.; Zeng, H.; Li, X.; Yan, Y.; Reiter, R.J.; He, C.; Shi, H. Identification, transcriptional and functional analysis of heat-shock protein 90s in banana (Musa acuminata L.) highlight their novel role in melatonin-mediated plant response to Fusarium wilt. J. Pineal Res. 2017, 62, 12367. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, 12379. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tan, D.X.; Reiter, R.J.; Shi, H. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 2015, 28, 15815. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal Melatonin: Cell Biology of Its Synthesis and of Its Physiological Interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Kandalepas, P.C.; Mitchell, J.W.; Gillette, M.U. Melatonin Signal Transduction Pathways Require E-Box-Mediated Transcription of Per1 and Per2 to Reset the SCN Clock at Dusk. PLoS ONE 2016, 11, e0157824. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Landete-Castillejos, T.; Zarazaga, L.; Garde, J.; Gallego, L. Seasonal changes in melatonin concentrations in female Iberian red deer (Cervus elaphus hispanicus). J. Pineal Res. 2003, 34, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Adamsson, M.; Laike, T.; Morita, T. Annual variation in daily light exposure and circadian change of melatonin and cortisol concentrations at a northern latitude with large seasonal differences in photoperiod length. J. Physiol. Anthropol. 2017, 36, 6. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Iigo, M.; Mizusawa, K.; Naruse, M.; Oishi, T.; Aida, K.; Tabata, M. Variations in Plasma Melatonin Levels of the Rainbow Trout (Oncorhynchus mykiss) under Various Light and Temperature Conditions. Zool. Sci. 2003, 20, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.R. Circadian Entrainment and Phase Shifting in Mammals with Melatonin. J. Biol. Rhythms 1997, 12, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, K.; Stehle, J.H. Melatonin synthesis in the human pineal gland: Advantages, implications, and difficulties. Chronobiol. Int. 2006, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, D.-X.; Jiang, D.; Liu, F. Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, K.; Zhou, X.; Xi, L.; Wang, Y.; Xu, H.; Pan, T.; Zou, Z. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci. Rep. 2017, 7, 4998. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.-P.; Scott, E.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous Melatonin Alleviates Cold Stress by Promoting Antioxidant Defense and Redox Homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Morley, N.J.; Lewis, J.W. Temperature stress and parasitism of endothermic hosts under climate change. Trends Parasitol. 2014, 30, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Burdick, N.C.; Chase, C.C., Jr.; Coleman, S.W.; Spiers, D.E. Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to a provocative immune challenge. Domest. Anim. Endocrinol 2012, 43, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Chen, L.; Poeggeler, B.; Manchester, C.; Reiter, R. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–63. [Google Scholar]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [PubMed]
- Daryani, A.; Montazeri, M.; Pagheh, A.S.; Sharif, M.; Sarvi, S.; Hosseinzadeh, A.; Reiter, R.J.; Hadighi, R.; Joghataei, M.T.; Ghaznavi, H.; et al. The potential use of melatonin to treat protozoan parasitic infections: A review. Biomed. Pharmacother. 2018, 97, 948–957. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, B.; Crespo, I.; Vallejo, D.; Álvarez, M.; Prieto, J.; González-Gallego, J.; Tuñón, M.J. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J. Pineal Res. 2014, 56, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Karbownik, M.; Reiter, R.J.; Tan, D.X.; Cuzzocrea, S.; Chiurazzi, P.; Cordaro, S.; Corona, G.; Trimarchi, G.; Barberi, I. Effects of Melatonin Treatment in Septic Newborns. Pediatr. Res. 2001, 50, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Marçola, M. Immune-pineal axis-acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Castellani, J.W.; Brenner, I.K.; Rhind, S.G. Cold exposure: Human immune responses and intracellular cytokine expression. Med. Sci. Sports Exerc. 2002, 34, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Groups | Animals | Beginning Date | Treatments | Termination Date |
|---|---|---|---|---|
| Control | 16 | 22 November 2015 | 12:12 h light/dark, 22 ± 2 °C, humidity (45–50%) | 22 December 2015 |
| Winter | 16 | 22 November 2015 | Local natural winter climate | 22 December 2015 |
| Summer | 16 | 22 May 2016 | Local natural summer climate | 22 June 2016 |
| Groups | Animals | Treatments | Duration | Termination |
|---|---|---|---|---|
| Col | 8 | 22 °C. | 72 h | 9–10 pm under red light (<5 w) |
| RTR | 8 | 22 rising to 38 °C during 30 min, last for 6 h, back to 25 °C | 72 h | 9–10 pm under red light (<5 w) |
| CHT | 8 | 38 °C | 72 h | 9–10 pm under red light (<5 w) |
| TRD | 8 | 22 declining to 4 °C during 30 min, last for 6 h, back to 25 °C. | 72 h | 9–10 pm under red light (<5 w) |
| CLT | 8 | 4 °C | 72 h | 9–10 pm under red light (<5 w) |
| Gene | Sequences (5′-3′) |
|---|---|
| AANAT | 225 bp |
| Forward | 5-GAGCAGCGGGAGGTTTGT-3 |
| Reverse | 5-ACTTGCGGTGCACGATGGAG-3 |
| ASMT/HIOMT | 245 bp |
| Forward | 5-ACAGCCTTTGACCTCTCACG-3 |
| Reverse | 5-ACCCGGGCAAGAATGAAGAG-3 |
| Actin | 383 bp |
| Forward | 5-CACTATTGGCAACGAGCGGTTC-3 |
| Reverse | 5-ACTTGCGGTGCACGATGGAG-3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, X.; Ma, S.; Xu, Y.; Xu, Y.; Guo, X.; Li, D. Association of Melatonin Production with Seasonal Changes, Low Temperature, and Immuno-Responses in Hamsters. Molecules 2018, 23, 703. https://doi.org/10.3390/molecules23030703
Xu X, Liu X, Ma S, Xu Y, Xu Y, Guo X, Li D. Association of Melatonin Production with Seasonal Changes, Low Temperature, and Immuno-Responses in Hamsters. Molecules. 2018; 23(3):703. https://doi.org/10.3390/molecules23030703
Chicago/Turabian StyleXu, Xiaoying, Xiaoyan Liu, Shuran Ma, Ya Xu, Ying Xu, Xiazhen Guo, and Dekui Li. 2018. "Association of Melatonin Production with Seasonal Changes, Low Temperature, and Immuno-Responses in Hamsters" Molecules 23, no. 3: 703. https://doi.org/10.3390/molecules23030703





