Charge-Controlled Synthetic Hyaluronan-Based Cell Matrices
Abstract
:1. Introduction
2. Results and Discussion
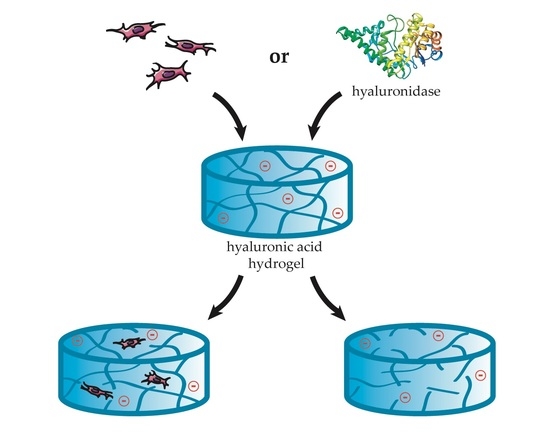
2.1. Synthetic ECM Model with Tunable Charge
2.2. Charge-Dependent Enzymatic Degradation
2.2.1. Hyaluronidase IV
2.2.2. Hyaluronate Lyase
2.2.3. Comparison
2.3. Charge-Dependent Protein Adsorption
2.3.1. Unspecific
2.3.2. Specific
2.4. Charge-Dependent Cell Attachment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. HA Hydrogel Formation and Physicochemical Characterization
4.2.1. HA Thiolation
4.2.2. HA Hydrogel Formation and Determination of Reacted Thiols
4.2.3. Mechanical Measurements
4.3. Calcultation of Negative Network Charge
4.4. Enzymatic Degradation
4.5. Protein Adsorption
4.6. Cell Cultivation on HA Hydrogels
4.7. Phase Contrast and Fluorescence Microscopy on HA Hydrogels
4.7.1. Live/Dead Staining
4.7.2. Microscopy Imaging
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. Molecular Biology of the Cell, 5th ed.; Garland: New York, NY, USA, 2007; pp. 1–15. [Google Scholar]
- Kuschel, C.; Steuer, H.; Maurer, A.N.; Kanzok, B.; Stoop, R.; Angres, B. Cell adhesion profiling using extracellular matrix protein microarrays. Curr. Biol. 2006, 40, 523–531. [Google Scholar] [CrossRef]
- Pelham, R.J.; Wang, Y.-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.; Leight, J.L.; Weaver, V.M. Demystifying the effects of a three-dimensional microenvironment in tissue morphogenesis. In Cell Mechanics; Methods in Cell Biology; Elsevier: London, UK, 2007; Volume 83, pp. 547–583. [Google Scholar]
- Butcher, J.T.; Nerem, R.M. Porcine aortic valve interstitial cells in three-dimensional culture: Comparison of phenotype with aortic smooth muscle cells. J. Heart Valve Dis. 2004, 13, 478–486. [Google Scholar] [PubMed]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Heher, P.; Hilborn, J.; Redl, H.; Ossipov, D.A. Hyaluronic acid-fibrin interpenetrating double network hydrogel prepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications. Acta Biomater. 2016, 38, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ranga, A.; Lutolf, M.P.; Hilborn, J.; Ossipov, D.A. Hyaluronic acid hydrogels formed in situ by transglutaminase-catalyzed reaction. Biomacromolecules 2016, 17, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Macosko, C.W.; Urry, D.W. Elastomeric polypentapeptides cross-linked into matrixes and fibers. Biomacromolecules 2001, 2, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.E.; Gonzalez, J.J.; Michaelson, R.P.; Glass, J.L.; Chock, D.A. Preliminary experience with new bioactive prosthetic material for repair of hernias in infected fields. Hernia 2002, 6, 171–174. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.; Cavanagh, B.; Unger, R.E.; Kirkpatrick, C.J.; O’Dea, S.; O’Brien, F.J.; Cryan, S.-A. The development of a tissue-engineered tracheobronchial epithelial model using a bilayered collagen-hyaluronate scaffold. Biomaterials 2016, 85, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-E.; Kim, S.-H.; Kim, H.D.; Yim, H.-G.; Bencherif, S.A.; Kim, T.-I.; Hwang, N.S. Extracellular matrix-based cryogels for cartilage tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Hegger, P.S.; Kupka, J.; Minsky, B.B.; Schädel, N.; Petri, N.; Laschat, S.; Boehm, H. Charge Matters: Modulating Secondary Interactions in Hyaluronan Hydrogels. ChemistrySelect 2017, 2, 7701–7705, Please see SI for experimental details on hydrogel synthesis and characterization. [Google Scholar] [CrossRef]
- Michael, A. On the addition of sodium acetacetic ether and analogous sodium compounds to unsaturated organic ethers. Am. Chem. J. 1887, 9, 115. [Google Scholar]
- Hagel, V.; Mateescu, M.; Southan, A.; Wegner, S.V.; Nuss, I.; Haraszti, T.; Kleinhans, C.; Schuh, C.; Spatz, J.P.; Kluger, P.J.; et al. Desmosine-inspired cross-linkers for hyaluronan hydrogels. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Alaish, S.M.; Yager, D.R.; Diegelmann, R.F.; Cohen, I.K. Hyaluronic acid metabolism in keloid fibroblasts. J. Pediatr. Surg. 1995, 30, 949–952. [Google Scholar] [CrossRef]
- Jackson, D.G. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol. Rev. 2009, 230, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Tille, J.-C.; Nisato, R.; Skobe, M. Lymphangiogenesis and tumor metastasis. Cell Tissue Res. 2003, 314, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Bailey, L.C.; Bailey, C.A.; Miller, R.T. Kinetic and mechanistic studies with bovine testicular hyaluronidase. Biochim. Et Biophys. Acta-Gen. Subj. 1994, 1200, 315–321. [Google Scholar] [CrossRef]
- Kelly, S.J.; Taylor, K.B.; Li, S.; Jedrzejas, M.J. Kinetic properties of streptococcus pneumoniae hyaluronate lyase. Glycobiology 2001, 11, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Frey, T. Nucleic acid dyes for detection of apoptosis in live cells. Cytometry Part A 1995, 21, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A. Ein neues Beleuchtungsverfahren für mikrophotographische Zwecke. Zeitschrift für wissenschaftliche Mikroskopie und für Mikroskopische Technik 1893, 4, 433–440. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |






| Degree of Thiolation (%) | Adsorbed Collagen (ng/mL) | Adsorbed Fibronectin (ng/mL) | Adsorbed Aggrecan (ng/mL) |
|---|---|---|---|
| 18 ± 1 | 0.001 ± 0.019 | −0.020 ± 0.011 | −0.011 ± 0.019 |
| 25 ± 4 | −0.015 ± 0.004 | −0.029 ± 0.006 | −0.024 ± 0.007 |
| 33 ± 2 | −0.020 ± 0.004 | −0.023 ± 0.012 | −0.031 ± 0.004 |
| Ratio of HA:DTP:EDCl | Batch of HA (Lot Lifecore) | Reaction Time | Degree of Thiolation |
|---|---|---|---|
| 1:1:1 | 025828 | 10 min | 18 ± 1% |
| 1:1:1 | 025828 | 15 min | 25 ± 1% |
| 1:1:1 | 025828 | 20 min | 33 ± 2% |
| Degree of Thiolation (%) | Crosslinker | Young’s Modulus (kPa) |
|---|---|---|
| 18 ± 1 | uncharged | 0.36 ± 0.02 |
| 18 ± 1 | charged | 2.57 ± 0.26 |
| 25 ± 4 | uncharged | 2.68 ± 0.13 |
| 25 ± 4 | charged | 3.65 ± 0.71 |
| 33 ± 2 | uncharged | 3.20 ± 0.12 |
| 33 ± 2 | charged | 6.57 ± 0.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegger, P.S.; Kupka, J.; Minsky, B.B.; Laschat, S.; Boehm, H. Charge-Controlled Synthetic Hyaluronan-Based Cell Matrices. Molecules 2018, 23, 769. https://doi.org/10.3390/molecules23040769
Hegger PS, Kupka J, Minsky BB, Laschat S, Boehm H. Charge-Controlled Synthetic Hyaluronan-Based Cell Matrices. Molecules. 2018; 23(4):769. https://doi.org/10.3390/molecules23040769
Chicago/Turabian StyleHegger, Patricia S., Julia Kupka, Burcu Baykal Minsky, Sabine Laschat, and Heike Boehm. 2018. "Charge-Controlled Synthetic Hyaluronan-Based Cell Matrices" Molecules 23, no. 4: 769. https://doi.org/10.3390/molecules23040769