Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities
Abstract
1. Introduction
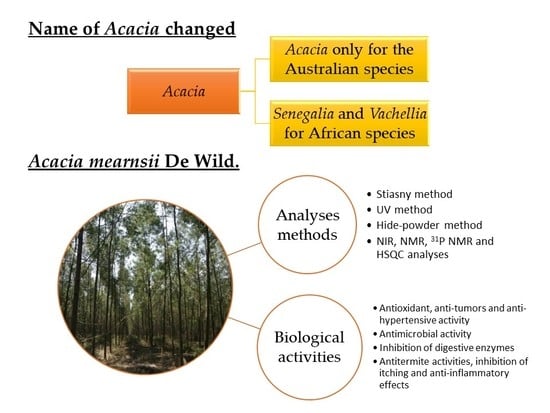
2. Taxonomy of Acacia
2.1. Acacia Mearnsii and Acacia Nilotica (Origin of the Genus “Acacia”)
2.2. Acacia Mearnsii De Wild.
3. Analyses Method of Wattle Tannin and Their Composition
3.1. Wattle Tannin Extracts and Tannin Analyses
3.1.1. Polyflavanoid Contents Analyzed Using the Stiasny Method for Wood Adhesives
3.1.2. Molecular Size Analysis of Wattle Tannin Extracts Using Ultrafiltration
3.1.3. Tannin Content of Wattle Tannin Using Hide-Powder Method for Leather Tanning
3.1.4. Tannin Analyses Using UV, Stiasny and Hide-Powder Methods
3.2. Estimation of Tannin Contents in the Bark Using NIR and NMR
3.3. Proanthocyanidin Composition of Wattle Tannin from A. mearnsii Bark
4. Biological Activities of Wattle Tannin
4.1. Antioxidant
4.2. Antimicrobial Activity
4.2.1. Antifungal Activity
4.2.2. Antibacterial Activity
4.2.3. Inhibitory Effects on Cyanobacteria
4.3. Inhibition of Enzymes
4.4. Anti-Obesity and Anti-Diabetes
4.5. Other Biological Activities
5. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Yazaki, Y. Utilization of Flavonoid Compounds from Bark and Wood: A Review. Nat. Prod. Commun. 2015, 10, 513–520. [Google Scholar] [PubMed]
- Stevens, P.F. “Fabaceae”. Available online: http://www.mobot.org/MOBOT/Research/APweb/orders/fabalesweb.htm#Fabaceae (accessed on 17 January 2018).
- Kewscience. Plants of the World online (/). Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:77089275-1 (accessed on 17 January 2018).
- Miller, P. The Gardeners Dictionary, 4th ed.London, UK, 1754. [Google Scholar]
- Ross, J.H. A survey of some of the pre-Linnean history of the genus Acacia. Bothalia 1980, 13, 95–110. [Google Scholar] [CrossRef][Green Version]
- Linnaeus, C. Species Plantarum, 1st ed.; Laurentius Salvius: Stockholm, Sweden, 1753. [Google Scholar]
- Orchard, A.E.; Maslin, B.R. Proposal to conserve the name Acacia (Leguminosae: Mimosoideae) with a new type. Taxan 2003, 52, 362–363. [Google Scholar] [CrossRef]
- Wattles-genus Acacia. Available online: http://www.anbg.gov.au/Acacia/ (accessed on 17 January 2018).
- Pedley, L. Derivation and dispersal of Acacia (Leguminosae), with particular reference to Australia, and the recognition of Senegalia and Racosperma. Bot. J. Linn. Soc. 1986, 92, 219–254. [Google Scholar] [CrossRef]
- Maslin, B.R.; Orchard, A.E.; West, J.G. Nomenclatural and classification history of Acacia (Leguminosae: Momosaoideae), and the implications of generic subdivision. Available online: http://worldwidewattle.com/infogallery/taxonomy/nomen-class.pdf (accessed on 17 January 2018).
- Carruthers, J.; Robin, L. Taxonomic imperialism in the battles for Acacia: Identity and science in South Africa and Australia. Trans. R. Soc. S. Afr. 2010, 65, 48–64. [Google Scholar] [CrossRef]
- Thiel, K.R.; Funk, V.A.; Iwatsuki, K.; Morat, P.; Peng, C.-I.; Raven, P.H.; Sarukhán, J.; Seberg, O. The controversy over the retypification of Acacia Mill. with an Australian type: A pragmatic view. Taxon 2011, 60, 194–198. [Google Scholar]
- Kyalangalilwa, B.; Boatwright, J.S.; Daru, B.H.; Maurin, O.; Van der Bank, M. Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Bot. J. Linn. Soc. 2013, 172, 500–523. [Google Scholar] [CrossRef]
- Rather, L.J.; Shahid-ul-Islam; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Dyer, C. New names for the African Acacia species in Vachellia and Senegalia. Southern For. J. For. Sci. 2014, 76. [Google Scholar] [CrossRef][Green Version]
- Boatwright, J.S.; Van der Bank, M.; Maurin, O. Name changes in African Acacia species. VELD FLORA 2014, 100, 33. [Google Scholar]
- De Wildeman, E.A.J. Vascular Plants APNI—Australian Plant Name Index. Available online: https://biodiversity.org.au/nsl/services/APNI?publication=Wildeman%2C+E.A.J.+De+%281925%29%2C+Plantae+Bequaertianae+3&search=true&advanced=true&display=apni (accessed on 17 January 2018).
- Tame, T.; Kodela, P.; Conn, B.; Hill, K. Available online: http://plantnet.rbgsyd.nsw.gov.au/cgi-bin/euctax.pl?/PlantNet/wattle=&name=Acacia+mearnsii (accessed on 17 January 2018).
- World Wide Wattle. Acacia mearnsii De Wild. Available online: http://worldwidewattle.com/speciesgallery/species-intro.php?id=17958 (accessed on 17 January 2018).
- Wiersum, K.F. Acacia mearnsii De Wild. In Plant Resources of South-East Asia No. 3. Dye and Tannin-producing Plants; Lemmens, R.H.M.J., Wulijarni-Soetjipto, N., Eds.; Pudoc/Prosea: Wageningen, The Netherlands, 1991; pp. 41–45. [Google Scholar]
- Turnbull, J.W.; Midgley, S.J.; Cossaltar, C. Tropical Acacias planted in Asia: An overview. In Recent Developments in Acacia Planting, Proceedings of the Third International Acacia Workshop, Hanoi, Vietnam, 27–31 October 1997; Turnbull, J.W., Compton, H.R., Pinyopusarerk, K., Eds.; Fores Science Institute of Vietnam: Hanoi, Vietnam.
- Roux, D.G. Study of the affinity of black wattle extract constituents. Part I. Affinity of polyphenols for swollen collagen and cellulose in water. J. Soc. Leather Trades’ Chem. 1955, 39, 80–91. [Google Scholar]
- Yazaki, Y.; Zheng, G.; Searle, S.D. Extractives Yields and Polyfavanoid Contents of Acacia mearnsii Barks in Australia. Aust. For. 1990, 53, 148–153. [Google Scholar] [CrossRef]
- Zheng, G.; Lin, Y.; Yazaki, Y. Comparing Molecular Size Distribution of Tannin Extracts from Acacia mearnsii Bark from Different Countries. Holzforshung 1988, 42, 407–408. [Google Scholar]
- Anon. Official Method of Analysis, 4th ed.; Society of Leather Trades’ Chemists: Hertfordshire, UK, 1965. [Google Scholar]
- Zheng, G.; Lin, Y.; Yazaki, Y. Bark tannin contents of Acacia mearnsii provenances and the relationship between the hide-powder and the Stiasny methods of estimation. Aust. For. 1991, 54, 209–211. [Google Scholar]
- Roux, D.G. Photometric methods of tannin analysis for black wattle tannin. J. Soc. Leather Technol. Chem. 1951, 35, 322–337. [Google Scholar]
- Yazaki, Y.; Gu, R.; Lin, Y.; Chen, W.; Nguyen, N.K. Analyses of Black Wattle (Acacia mearnsii) Tannins-Relationships Among the Hide-Powder, the Stiasny and the Ultra-Violet (UV) Methods. Holzforschung 1993, 47, 57–61. [Google Scholar] [CrossRef]
- Donkin, M.J.; Pearce, J. Tannin analysis by near infrared spectroscopy. J. Soc. Leather Technol. Chem. 1995, 79, 8–11. [Google Scholar]
- Schimleck, L.R.; Yazaki, Y. Analysis of Black Wattle (Acacia mearnsii De Wild.) Bark by Near Infrared Spectroscopy. Holzforschung 2003, 57, 527–532. [Google Scholar] [CrossRef]
- Menezes, C.M.; Ben da Costa, A.; Renner, R.R.; Bastos, L.F.; Ferrão, M.F.; Dressler, V.L. Direct determination of tannins in Acacia mearnsii bark using near-infrared spectroscopy. Anal. Methods 2014, 6, 8299–8305. [Google Scholar] [CrossRef]
- Grasel, F.S.; Marcelo, M.C.A.; Ferrão, M.F. Development of an inexpensive, practical and non-destructive methodology based on digital images from a scanner for the classification of commercial tannins from Acacia mearnsii. Anal. Methods 2017, 9, 3977–3982. [Google Scholar] [CrossRef]
- Reid, D.G.; Bonnet, S.L.; Kemp, G.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 4: solid state 13C-NMR as a tool for in situ analysis of proanthocyanidin tannins, in heartwood and bark of Quebracho and Acacia, and related species. Phytochemistry 2013, 94, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Botha, J.J.; Ferreira, D.; Roux, D.G. Condensed Tannins: Direct Synthesis, Structure, and Absolute Configuration of Four Biflavonoids from Black Wattle Bark (‘Mimosa’) Extract. J. Chem. Soc. Chem. Commun. 1978, 700–702. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G. Condensed Tannins: Condensation Mode and Sequence during Formation of Synthetic and Natural Triflavonoids. J. Chem. Soc. Chem. Commun. 1979, 510–512. [Google Scholar] [CrossRef]
- Shen, Z.; Yu, Q.; Chen, X.; Shen, H.; Xiao, Z. Study on black wattle tannins. III. Isolation and identification of proanthocyanidins and their 13C-NMR characteristics. Linchan Huaxue Yu Gongye 1991, 11, 85–95. [Google Scholar]
- Roux, D.G. Review Article: Recent advances in the chemistry and chemical utilization of the natural condensed tannins. Phytochemistry 1972, 11, 1219–1230. [Google Scholar] [CrossRef]
- Saayman, H.M.; Roux, D.G. The origins of tannins and flavonoids in black-wattle barks and heartwoods, and their associated “non-tannin” components. Biochem. J. 1965, 97, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Kusano, R.; Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y.; Kouno, I. α-Amylase and lipase inhibitory activity and structural characterization of Acacia bark proantocyanidins. J. Nat. Prod. 2011, 74, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Yasuta, Y.; Ohi, H. Structure elucidation of condensed tannins from barks by pyrolysis/gas chromatography. Holzforschung 2003, 57, 145–149. [Google Scholar] [CrossRef]
- Venter, P.B.; Senekal, N.D.; Kemp, G.; Amra-Jordaan, M.; Khan, P.; Bonnet, S.L.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 3: The chemical composition of wattle (Acacia mearnsii) bark extract. Phytochemistry 2012, 83, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Lange, H.; Bianchetti, G. Detailed chemical composition of condensed tannins via quantitative (31) P NMR and HSQC analyses: Acacia catechu, Schinopsis balansae, and Acacia mearnsii. J. Nat. Prod. 2016, 79, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F. Investigation on biological activities of proanthocyanidins from black wattle bark. Chem. Ind. For. Prod. 2007, 27, 43–48. [Google Scholar]
- Shen, X.; Wang, Y.; Wang, F. Characterisation and biological activities of proanthocyanidins from the barks of Pinus massonian and Acacia mearnsii. Nat. Prod. Res. 2010, 24, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Niu, H.; Xue, X.; Li, J.; Li, C. Robinetinidol-(4β→8)-epigallocatechin 3-O-gallate, a galloyl dimer prorobinetinidin from Acacia mearnsii De Wild, effectively protects human neuroblastoma SH-SY5Y cells against acrolein-induced oxidative damage. J. Alzheimer’s Dis. 2010, 2, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, G.; He, L.; Wang, F. Preparation and antioxidant activity of proanthocyanidins dimers from Acacia mearnsii De Willd. Chem. Ind. For. Prod. 2017, 37, 135–140. [Google Scholar]
- Ikarashi, N.; Toda, T.; Hatakeyama, Y.; Kusunoki, Y.; Kon, R.; Mizukami, N.; Kaneko, M.; Ogawa, S.; Sugiyama, K. Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rat. Int. J. Mol. Sci. 2018, 19, 700. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Tsunoda, T.; Ono, K.; Yazaki, Y.; Tongi, L.Y.; Lawson, F.; Uhlherr, P.H.T. Active Oxygen Scavenger Prepared from Acacia Plant Bark, and Composition Made from the Same. Jp. Patent 2004352639, 16 December 2004. [Google Scholar]
- Nakamoto, Y.; Ono, K. Antioxidant Composition Containing Component Originating in the Bark of Tree Belonging to the Genus Acacia. Jp. Patent 2006306803, 9 November 2006. [Google Scholar]
- Nakamoto, Y.; Ono, K. Composition for Preventing and/or Treating Tumor Containing Component Originating in the Bark of Tree Belonging to the Genus Acacia. Jp. Patent 2006306801, 9 November 2006. [Google Scholar]
- Wiid, M.N. Anti-Oxidant Compositions. WO Patent 2009126976, 15 October 2009. [Google Scholar]
- Ohara, S.; Suzuki, K.; Ohira, T. Condensed tannins from Acacia mearnsii and their biological activities. Mokuzai Gakkaishi. 1994, 40, 1363–1374. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Pharmacological assessment of the medicinal potential of Acacia mearnsii De Wild.: antimicrobial and toxicity activities. Int. J. Mol. Sci. 2012, 13, 4255–4267. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Vargas, Á.; Fronza, N.; dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloids Surf. B: Biointerfaces 2017, 151, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Timotheo, C.A.; Lauer, C.M., Jr. Toxicity of vegetable tannin extract from Acacia mearnsii in Saccharomyces cerevisiae. Int. J. Environ. Sci. Technol. 2017, 15, 659–664. [Google Scholar] [CrossRef]
- Morandi, L.A.P.; Morandi, J.L.P. Antifungal Composition Based on Plant Extracts for Treatment of Green Wood. Br. Patent 2006001476, 18 December 2007. [Google Scholar]
- Zhang, L.; Li, J.; Liu, Y. Compounded Bacteriostatic Agent Containing Plant Polyphenol. Cn. Patent 1957697, 9 May 2007. [Google Scholar]
- Smith, A.H.; Imlay, J.A.; Mackie, R.I. Increasing the oxidative stress response allows Escherichia coli to overcome inhibitory effects of condensed tannins. Appl. Environ. Microbiol. 2003, 69, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.G.; Smith, A.H.; Sundset, M.A.; Mackie, R.I. The BaeSR two-component regulatory system mediates resistance to condensed tannins in Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 535–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olajuyigbe, O.O.; Afolayan, A.J. In vitro antibacterial and time-kill assessment of crude methanolic stem bark extract of Acacia mearnsii De Wild against bacteria in shigellosis. Molecules 2012, 17, 2103–2118. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, O.O.; Afolayan, A.J. A comparative effect of the alcoholic and aqueous extracts of Acacia mearnsii De Wild on protein leakage, lipid leakage and ultrastructural changes in some selected bacterial strains as possible mechanisms of antibacterial action. J. Pure Appl. Microbiol. 2014, 8, 1243–1257. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Synergistic interactions of methanolic extract of Acacia mearnsii De Wild. with antibiotics against bacteria of clinical relevance. Int. J. Mol. Sci. 2012, 13, 8915–8932. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, O.O.; Coopoosamy, R.M. Influence of first-line antibiotics on the antibacterial activities of acetone stem bark extract of Acacia mearnsii De Wild. against drug-resistant bacterial isolates. Evid. Based Complement. Alternat. Med 2014, 2014, 423751. [Google Scholar] [CrossRef] [PubMed]
- Klug, T.V.; Novello, J.; Laranja, D.C.; Aguirre, T.A.S.; de Oliveira Rios, A.; Tondo, E.C.; dos Santos, R.P.; Bender, R.J. Effect of tannin extracts on biofilms and attachment of Escherichia coli on lettuce leaves. Food Bioprocess Technol. 2016, 10, 275–283. [Google Scholar] [CrossRef]
- Zhou, L.; Bi, Y.; Jiang, L.; Wang, Z.; Chen, W. Effect of black wattle (Acacia mearnsii) extract on blue-green algal bloom control and plankton structure optimization: a field mesocosm experiment. Water Env. Res. 2012, 84, 2133–2142. [Google Scholar] [CrossRef]
- Luo, K.; Liu, D.; Zhou, L.; Chen, W. Allelopathic inhibitory effect of the Acacia mearnsii extracts on Microcystis aeruginosa. J. Shenzhen Univ. Sci. Eng. 2014, 31, 205–209. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Liu, D.; Zhu, Q.; Chen, W. Inhibitory mechanisms of Acacia mearnsii extracts on the growth of Microcystis aeruginosa. Water Sci. Technol. 2015, 71, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Mitsunaga, T. Tyrosinase inhibitory activity of proanthocyanidins from woody plants. J. Wood Sci. 2003, 49, 461–465. [Google Scholar] [CrossRef]
- Abe, I.; Mitsunaga, T.; Takagi, K.; Shimomura, K. Preparation for External Use for Skin/Novel Use of Extract Isolated from Bark of Larch, Acacia or Duramen of Schinopsis lorentsii as Skin Whitening Agent. Jp. Patent 10025238, 27 January 1998. [Google Scholar]
- Cuirong, L. Skin Deep-Clean Facial Cream for Suppressing Tyrosinase and Decomposing Melanin. Cn. Patent 106963681, 21 July 2017. [Google Scholar]
- Da Silva, S.M.; Koehnlein, E.A.; Bracht, A.; Castoldi, R.; de Morais, G.R.; Baesso, M.L.; Peralta, R.A.; de Souza, C.G.M.; de Sá -Nakanishi, A.B.; Peralta, R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kusano, R.; Ogawa, S.; Yazaki, Y.; Tanaka, T. Characterization of the α-amylase inhibitory activity of oligomeric proanthocyanidins from Acacia mearnsii Bark Extract. Nat. Prod. Commun. 2016, 11, 1851–1854. [Google Scholar]
- Kato, C.G.; de Almeida Gonçalves, G.; Peralta, R.A.; Seixas, F.A.V.; de Sá-Nakanishi, A.B.; Bracht, L.; Comar, J.F.; Bracht, A.; Peralta, R.M. Inhibition of α-amylases by condensed and hydrolysable tannins: focus on kinetics and hypoglycemic actions. Enzyme Res. 2017, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Grace, M.H.; Esposito, D.; Komarnytsky, S.; Wang, F.; Lila, M.A. Polyphenols isolated from Acacia mearnsii bark with anti-inflammatory and carbolytic enzyme inhibitory activities. Chin. J. Nat. Med. 2017, 15, 816–824. [Google Scholar] [CrossRef]
- Ikarashi, N.; Toda, T.; Okaniwa, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Anti-obesity and anti-diabetic effects of Acacia polyphenol in obese diabetic KKAy mice fed high-fat diet. Evid.-Based Complement. Alternat. Med. 2011, 2011, 952031. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Takeda, R.; Ito, K.; Ochiai, W.; Sugiyama, K. The inhibition of lipase and glucosidase activities by Acacia polyphenol. Evid.-Based Complement. Alternat. Med. 2011, 2011, 272075. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Ono, K. Hypoglycemic Composition Containing Component Originating in the Bark of Tree Belonging to the Genus Acacia. Jp. Patent 2006232781, 7 September 2006. [Google Scholar]
- Nakamoto, Y.; Ono, K. Antiobesity Composition Containing Component Originating in the Bark of Tree Belonging to the Genus Acacia. Jp. Patent 2006232782, 7 September 2006. [Google Scholar]
- Fava, F.J.; Monteiro De Barros, N.; Stumpp, E.; Ramao Marceli, F., Jr. Aqueous Extract to Repel or Exterminate Termites. WO Patent 2006021064, 2 March 2006. [Google Scholar]
- Fermandes, R.M., Jr.; Fava, F.J.; Gobatto, V.; Monteiro de, B.N.; Specht, A. Acacia mearnsii Bark Extract as Insecticide. Br. Patent 2006002039, 8 January 2008. [Google Scholar]
- Ikarashi, N.; Sato, W.; Toda, T.; Ishii, M.; Ochiai, W.; Sugiyama, K. Inhibitory effect of polyphenol-rich fraction from the bark of Acacia mearnsii on itching associated with allergic dermatitis. Evid.-Based Complement. Alternat. Med. 2012, 2012, 120389. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Hattori, F.; Ito, S.; Yamada, S.; Takagi, K.; Shimomura, K. Histamine Liberation Suppressing Agent/Histamine Release Inhibitor Containing Plant Extracts for Relieving. Jp. Patent 2001048766, 20 February 2001. [Google Scholar]
- Nakamoto, Y.; Ono, K. Composition for Preventing and/or Treating Itching Containing Component Originating in the Bark of Trees Belonging to Acacia. Jp. Patent 2006306802, 9 November 2006. [Google Scholar]
| No. | Extracts | Tested Bacterial Isolates | Growth or Inhibition | Reference |
|---|---|---|---|---|
| 1 * | Water soluble fraction (AW) from 70% acetone-water soluble extract | Coriolus versicolor Tyromyces palustris | No antifungal activity at 0.01–0.25% concentrations | [52] |
| Ethyl acetate soluble fraction (EA) from 70% acetone-water soluble extract | C. versicolor T. Palustris | Very mild activities at 0.1–0.25% concentration | ||
| Crude acetone extract | Candida krusei Candida albicans Candida rugosa Candida glabrata ATCC 2001 Absidia corymbifera Fusarium sporotrichioides Trichophyton tonsurans Trichophyton mucoides ATCC 201382 Aspergillus niger Aspergillus terreus Aspergillus flavus | MIC values were fungal isolates (625–5000) μg/mL | [53] | |
| Tannins | A. niger ATCC 9642 Candida sp. ATCC 14053 | Weak inhibition activity | [54] | |
| 2 * | Aqueous extract | Saccharomyces cerevisiae BY4741 S. cerevisiae Δ gsh1 | In the poisoner quantitative drop test, toxicological effects from a concentration of 4.20 mg/L | [55] |
| Crude acetone extract | Escherichia coli ATCC 25922 Bacillus cereus ATCC 10702 Proteus vulgaris KZN Serratia marcescens ATCC 9986 Pseudomonas aeruginosa ATCC 19582 Enterococcus faecalis KZN Klebsiella pneumoniae ATCC 10031 P. vulgaris CSIR 0030 Bacillus pumilus ATCC 14884 K. pneumoniae KZN Staphylococcus aureus OK1 Salmonella typhi ATCC 13311 | MIC values were Gram-positive bacteria (78.1–312.5) μg/mL, Gram-negative bacteria (39.1–625) μg/mL | [53] | |
| Tannins | S. aureus | Strong inhibition activity | [54] | |
| Aqueous extract | E. coli BW13711 E. coli TA4131 | No growth in 0.1% wattle tannin extract medium | [58] | |
| E. coli WTT1 E. coli TA4110 | No growth in 0.15% wattle tannin extract medium | |||
| Aqueous extract | E. coli BW13711 E. coli ΔBaeSR13711 | Growth of the E. coli ΔBaeSR mutant reached behind stationary phase compared to that of E. coli BW13711 in the presence of tannins | [59] | |
| Crude methanol extract | E. coli ATCC 8739 K. pneumoniae ATCC 10031 B. pumilus ATCC 14884 P. vulgaris ATCC 6830 Acinetobacter calcoaceticus A. calcoaceticus anitratis CSIR P. vulgaris CSIR 0030 Shigella flexneri KZN S. typhi ATCC 13311 Micrococcus luteus E. faecalis KZN S. aureus OK2b S. aureus OK1 S. aureus OK3 | Minimum inhibitory concentration (MIC) values were gram-negative (0.0391–0.3125) mg/mL and gram-positive bacteria (0.0781–0.625) mg/mL | [60] | |
| Acetone, methanol and aqueous extracts | E. coli S. aureus B. pumilus P. vulgaris S. flexneri | Extracts caused the disruption of the cytoplasmic membranes of the bacterial cells. | [61] | |
| Methanol extract | S. aureus ATCC 6538 E. faecalis ATCC 29212 E. faecalis ATCC 29212 E. coli ATCC 25922 Bacillus subtilis KZN P. vulgaris KZN E. faecalis KZN Enterobacter cloacae ATCC 13047 K. pneumoniae (ATCC 10031) P. vulgaris ATCC 6830 Shigella sonnei ATCC 29930 | Synergetic, indifferent and antagonistic interactions were differences depending on combination between bacterial types and antibiotics agent types with the extract | [62] | |
| 3 * | Acetone extract | E. coli ATCC 25922 B. cereus ATCC 10702 P. aeruginosa ATCC 19582 S. marcescens ATCC 9986 E. faecalis KZN S. aureus ok1 S. flexneri KZN M. luteus P. vulgaris CSIR 0030 S. typhi ATCC 13311 | Synergetic, additive, indifference and antagonism interactions were differences depending on combination between bacterial types and antibiotics agent types with the extract | [63] |
| Tannin extract | E. coli ATCC 25922 | Tannin extract is capable of reducing the counts of E. coli adhered to and under biofilm formation on lettuce leaves. | [64] | |
| Aqueous extract | Microcystis aeruginosa | Black wattle extract inhibits algal blooms in a short-term test and the extract maintains water quality and prevents algal blooms in a long-term test. | [65] | |
| Extract | M. aeruginosa | Extract damage to the ultrastructure of the algal cell and decrease algal cells and chlorophyll-a. | [66] | |
| Aqueous extract | M. aeruginosa | Expression of photosynthesis-related genes was remarkably reduced in the presence of the extract. | [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules 2018, 23, 837. https://doi.org/10.3390/molecules23040837
Ogawa S, Yazaki Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules. 2018; 23(4):837. https://doi.org/10.3390/molecules23040837
Chicago/Turabian StyleOgawa, Sosuke, and Yoshikazu Yazaki. 2018. "Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities" Molecules 23, no. 4: 837. https://doi.org/10.3390/molecules23040837
APA StyleOgawa, S., & Yazaki, Y. (2018). Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules, 23(4), 837. https://doi.org/10.3390/molecules23040837