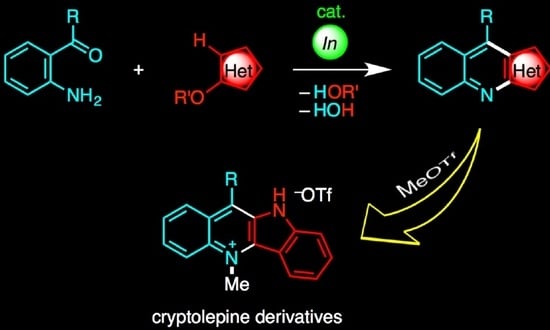
Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: Synthesis of Heteroaryl[b]quinolines and Subsequent Transformation to Cryptolepine Derivatives
Abstract
:1. Introduction
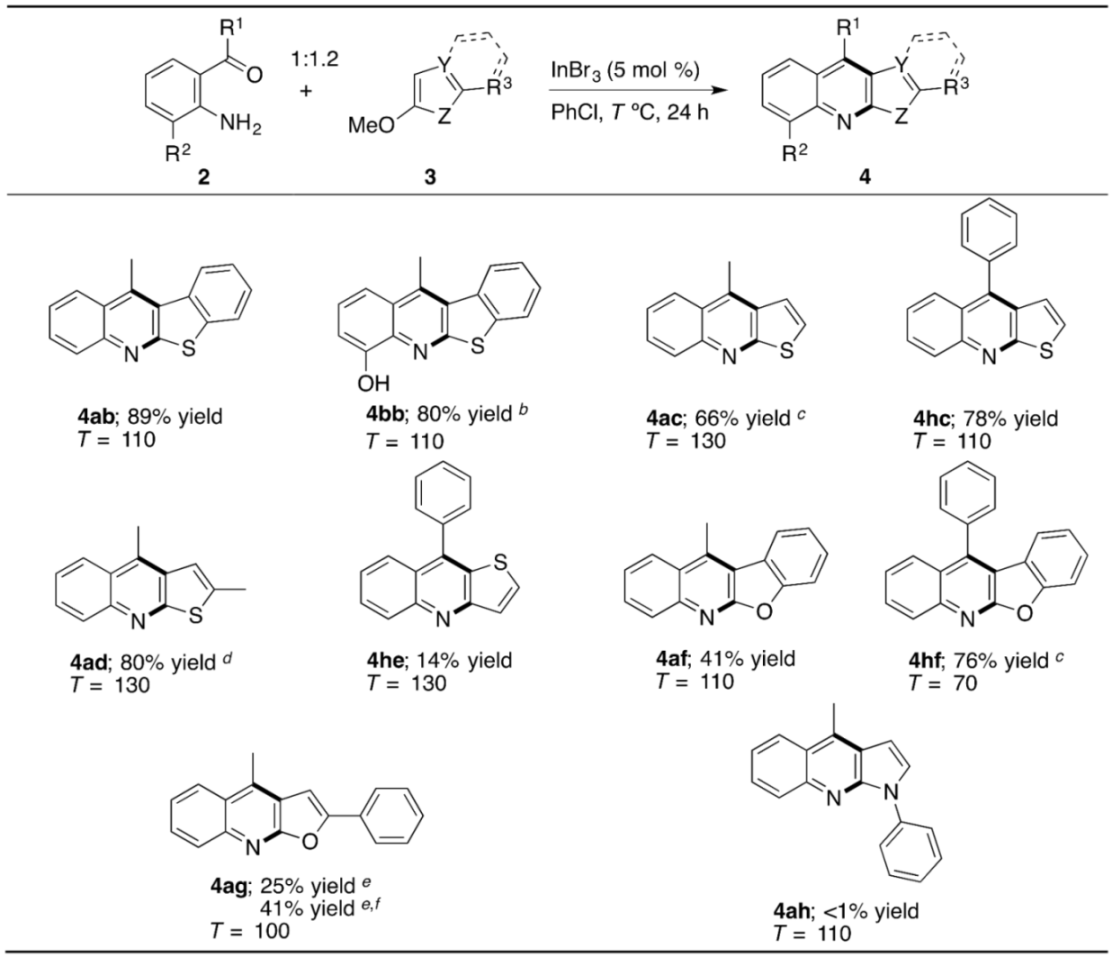
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Substrates
3.2.1. Synthesis of 1-(2-Amino-5-chlorophenyl)-2,2,2-trifluoroethanone (2g): Removal of HCl and H2O from 2g–HCl–H2O
3.2.2. Synthesis of 1-[2-(Dimethylamino)phenyl]-2-methyl-1-propanone (Me2-2e)
3.3. Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: An Experimental Procedure Exemplified by the Synthesis of 4aa
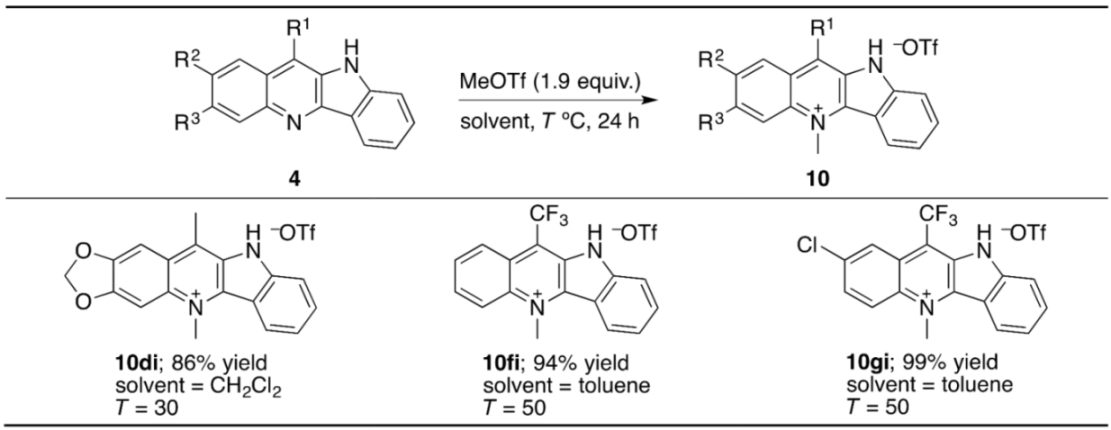
3.4. N-Methylation of Indolo[3,2-b]quinolines with MeOTf: An Experimental Procedure Exemplified by the Synthesis of 10fi
3.5. An Experimental Procedure for the Synthesis of 11fi by Neutralizing 10fi
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Lal, B.; Bhise, N.B.; Gidwani, R.M.; Lakdawala, A.D.; Joshi, K.; Patvardhan, S. Isolation, synthesis and biological activity of Evolitrine and analogs. ARKIVOC 2005, 2005, 77–97. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Loke, P.L.; Malone, J.F.; McRoberts, W.C.; Hamilton, J.T.G. Synthesis, structure and stereochemistry of quinoline alkaloids from Choisya ternata. Org. Biomol. Chem. 2007, 5, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, S.; Wan, S.; Ren, S.; Li, W.; Jiang, T. Efficient synthesis of jusbetonin, an indolo[3,2-b]quinoline glycoside, and its derivatives. Carbohydr. Res. 2009, 344, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Takeuchi, Y.; Hashigaki, K.; Ikeda, Y.; Chang, M.R.; Takeuchi, K.; Matsushima, M.; Tsuruo, T.; Tashiro, T.; Tsukagoshi, S.; et al. Synthesis and Antitumor Activity of Fused Tetracyclic Quinoline Derivatives. 1. J. Med. Chem. 1989, 32, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Tseng, C.-H.; Chen, Y.-L.; Lu, C.-M.; Kao, C.-L.; Wu, M.-H.; Tzeng, C.-C. Identification of benzofuro[2,3-b]quinoline derivatives as a new class of antituberculosis agents. Eur. J. Med. Chem. 2010, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.T.; Ajay, A.K.; Bhat, M.K.; Parameswaran, P.S.; Tilve, S.G. Iodine catalyzed one-pot synthesis of chloro-substituted linear and angular indoloquinolines and in vitro antiproliferative activity study of different indoloquinolines. Med. Chem. Res. 2013, 22, 88–93. [Google Scholar] [CrossRef]
- Du, G.; Huang, S.-M.; Zhai, P.; Chen, S.-B.; Hua, W.-Z.; Tan, J.-H.; Ou, T.-M.; Huang, S.-L.; Li, D.; Gu, L.-Q.; et al. Synthesis and evaluation of new BODIPY-benzofuroquinoline conjugates for sensitive and selective DNA detection. Dyes Pigment. 2014, 107, 97–105. [Google Scholar] [CrossRef]
- Sokhanvar, M.; Pordel, M. Synthesis, spectroscopic characterization and DFT calculations of a new highly fluorescent heterocyclic system: Imidazo[4,5-a]quinindoline. ARKIVOC 2014, 2014, 328–341. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, X.; Zhao, N.; Xu, S.; An, Z.; Zhuang, X.; Lan, Z.; Wen, L.; Wan, X. Reductive Ring Closure Methodology toward Heteroacenes Bearing a Dihydropyrrolo[3,2-b]pyrrole Core: Scope and Limitation. J. Org. Chem. 2014, 79, 11339–11348. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, P.; Thiruvengadam, T.K.; Ramasamy, K. Synthese von Furo[2,3-b]chinolinen. Monatshefte Chem. 1977, 108, 725–733. [Google Scholar] [CrossRef]
- Gee, M.B.; Lee, W.J.; Yum, E.K. Synthesis of Pyrrolo[2,3-b]quinolines by Palladium-catalyzed Heteroannulation. Bull. Korean Chem. Soc. 2003, 24, 1193–1196. [Google Scholar] [CrossRef]
- Dutta, B.; Some, S.; Ray, J.K. Thermal cyclization of 3-arylamino-3-(2-nitrophenyl)-propenal Schiff base hydrochlorides followed by triethyl phosphite mediated deoxygenation: A facile synthesis of quindolines. Tetrahedron Lett. 2006, 47, 377–379. [Google Scholar] [CrossRef]
- Vera-Luque, P.; Alajarín, R.; Alvarez-Builla, J.; Vaquero, J.J. An Improved Synthesis of α-Carbolines under Microwave Irradiation. Org. Lett. 2006, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Aillaud, I.; Bossharth, E.; Conreaux, D.; Desbordes, P.; Monteiro, N.; Balme, G. A Synthetic Entry to Furo[2,3-b]pyridin-4(1H)-ones and Related Furoquinolinones via Iodocyclization. Org. Lett. 2006, 8, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.N.S.S.P.; Srihari, E.; Ravinder, M.; Kumar, K.P.; Murthy, U.S.N.; Rao, V.J. DBU Promoted Facile Synthesis of New Thieno[2,3-b]pyridine/quinoline Derivatives and Their Antimicrobial Evaluation. J. Heterocycl. Chem. 2013, 50, E131–E135. [Google Scholar] [CrossRef]
- Bogányi, B.; Kámán, J. A concise synthesis of indoloquinoline skeletons applying two consecutive Pd-catalyzed reactions. Tetrahedron 2013, 69, 9512–9519. [Google Scholar] [CrossRef]
- Wu, B.; Yoshikai, N. Versatile Synthesis of Benzothiophenes and Benzoselenophenes by Rapid Assembly of Arylzinc Reagents, Alkynes, and Elemental Chalcogens. Angew. Chem. Int. Ed. 2013, 52, 10496–10499. [Google Scholar] [CrossRef] [PubMed]
- Barl, N.M.; Sansiaume-Dagousset, E.; Monzón, G.; Wagner, A.J.; Knochel, P. Preparation and Reactions of Heteroarylmethylzinc Reagents. Org. Lett. 2014, 16, 2422–2425. [Google Scholar] [CrossRef] [PubMed]
- Iaroshenko, V.O.; Gevorgyan, A.; Mkrtchyan, S.; Grigoryan, T.; Movsisyan, E.; Villinger, A.; Langer, P. Regioselective Direct Arylation of Fused 3-Nitropyridines and Other Nitro-Substituted Heteroarenes: The Multipurpose Nature of the Nitro Group as a Directing Group. ChemCatChem 2015, 7, 316–324. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, S. Rh(III)-catalyzed 7-azaindole synthesis via C–H activation/annulative coupling of aminopyridines with alkynes. Chem. Commun. 2015, 51, 11202–11205. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, B.; Song, X.; Xie, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Synthesis of Quinolines from Propargyl Silyl Ethers and Anthranils through the Umpolung of a Gold Carbene Carbon. Angew. Chem. Int. Ed. 2016, 55, 12688–12692. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.D.; Garud, D.R.; Udagawa, T.; Koketsu, M. Synthesis of thieno[2,3-b]quinoline and selenopheno[2,3-b]quinoline derivatives via iodocyclization reaction and a DFT mechanistic study. Org. Biomol. Chem. 2018, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bierer, D.E.; Dubenko, L.G.; Zhang, P.; Lu, Q.; Imbach, P.A.; Garofalo, A.W.; Phuan, P.-W.; Fort, D.M.; Litvak, J.; Gerber, R.E.; et al. Antihyperglycemic Activities of Cryptolepine Analogues: An Ethnobotanical Lead Structure Isolated from Cryptolepis sanguinolenta. J. Med. Chem. 1998, 41, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Rádl, S.; Konvička, P.; Váchal, P. A New Approach to the Synthesis of Benzofuro[3,2-b]quinolines, Benzothieno[3,2-b]quinolines and Indolo[3,2-b]quinolines. J. Heterocycl. Chem. 2000, 37, 855–862. [Google Scholar] [CrossRef]
- Strekowski, L.; Lee, H.; Lin, S.-Y.; Czarny, A.; Van Derveer, D. Synthesis and Conformation of 2-Aminophenyldiarylperfluoroalkylmethanes (Molecular Propellers). J. Org. Chem. 2000, 65, 7703–7706. [Google Scholar] [CrossRef] [PubMed]
- Tugusheva, N.Z.; Ryabova, S.Y.; Solov’eva, N.P.; Anisimova, O.S.; Granik, V.G. Investigation of the Reaction of N-Acetylindoxyl with substituted anilines. Synthesis of derivatives of indolo[3,2-b]quinolines. Chem. Heterocycl. Compd. 2001, 37, 885–893. [Google Scholar] [CrossRef]
- Patteux, C.; Levacher, V.; Dupas, G. A Novel Traceless Solid-Phase Friedländer Synthesis. Org. Lett. 2003, 5, 3061–3063. [Google Scholar] [CrossRef] [PubMed]
- Engqvist, R.; Bergman, J. An improved synthesis of neocryptolepine. Org. Prep. Proced. Int. 2004, 36, 386–390. [Google Scholar] [CrossRef]
- Iaroshenko, V.O.; Wang, Y.; Zhang, B.; Volochnyuk, D.; Sosnovskikh, V.Y. Facile Synthesis of Fluorinated Benzofuro- and Benzothieno[2,3-b]pyridines, α-Carbolines and Nucleosides Containing the α-Carboline Framework. Synthesis 2009, 2393–2402. [Google Scholar] [CrossRef]
- Ali, S.; Li, Y.-X.; Anwar, S.; Yang, F.; Chen, Z.-S.; Liang, Y.-M. One-Pot Access to Indolo[2,3-b]quinolines by Electrophile-Triggered Cross-Amination/Friedel–Crafts Alkylation of Indoles with 1-(2-Tosylaminophenyl)ketones. J. Org. Chem. 2012, 77, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Vaghei, R.; Malaekehpoor, S.M. N-Bromosuccinimide as an efficient catalyst for the synthesis of indolo[2,3-b]quinolines. Tetrahedron Lett. 2012, 53, 4751–4753. [Google Scholar] [CrossRef]
- Lian, Y.; Hummel, J.R.; Bergman, R.G.; Ellman, J.A. Facile Synthesis of Unsymmetrical Acridines and Phenazines by a Rh(III)-Catalyzed Amination/Cyclization/Aromatization Cascade. J. Am. Chem. Soc. 2013, 135, 12548–12551. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, H.; Yu, Y.; Wu, W.; Jiang, H. Palladium-Catalyzed Intermolecular Aerobic Oxidative Cyclization of 2-Ethynylanilines with Isocyanides: Regioselective Synthesis of 4-Halo-2-aminoquinolines. J. Org. Chem. 2013, 78, 10319–10328. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhou, Y.; Huang, H.; Yang, Y.; Liu, Y.; Li, Y. Copper-Catalyzed Synthesis of Substituted Quinolines via C–N Coupling/Condensation from ortho-Acylanilines and Alkenyl Iodides. J. Org. Chem. 2015, 80, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, M.; Wojciechowski, K. Simple synthesis 11-substituted norcryptotackieine derivatives. RSC Adv. 2015, 5, 94296–94303. [Google Scholar] [CrossRef]
- Yan, Z.; Wan, C.; Wan, J.; Wang, Z. An efficient iron-promoted synthesis of 6H-indolo[2,3-b]quinolines and neocryptolepine derivatives. Org. Biomol. Chem. 2016, 14, 4405–4408. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, B. Tandem Rh(III)-Catalyzed C–H Amination/Annulation Reactions: Synthesis of Indoloquinoline Derivatives in Water. Org. Lett. 2016, 18, 2820–2823. [Google Scholar] [CrossRef] [PubMed]
- Senadi, G.C.; Dhandabani, G.K.; Hu, W.-P.; Wang, J.-J. Metal-free annulation/aerobic oxidative dehydrogenation of cyclohexanones with o-acylanilines: Efficient syntheses of acridines. Green Chem. 2016, 18, 6241–6245. [Google Scholar] [CrossRef]
- Janni, M.; Thirupathi, A.; Arora, S.; Peruncheralathan, S. Chemoselective Ullmann coupling at room temperature: A facile access to 2-aminobenzo[b]thiophenes. Chem. Commun. 2017, 53, 8439–8442. [Google Scholar] [CrossRef] [PubMed]
- Obata, N.; Mizuno, H.; Koitabashi, T.; Takizawa, T. The Cycloaddition Reaction of Isonitrile to Ketenimine Formed by the Reaction of Isonitrile with Carbene. Bull. Chem. Soc. Jpn. 1975, 48, 2287–2293. [Google Scholar] [CrossRef]
- Szmuszkovicz, J.; Baczynskyj, L.; Chidester, C.C.; Duchamp, D.J. Reaction of 2-Amino-7-chloro-5-phenyl-3H-[1,4]benzodiazepine with 1,3-Dicarbonyl Compounds. J. Org. Chem. 1976, 41, 1743–1747. [Google Scholar] [CrossRef]
- Sunder, S.; Peet, N.P. Synthesis of Benzofuro[3,2-b]quinoline-6(11H)one and Derivatives. J. Heterocycl. Chem. 1978, 15, 1379–1382. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Chang, M.-R.; Hashigaki, K.; Tashiro, T.; Tsuruo, T.; Tsukagoshi, S.; Yamato, M. Synthesis and Antitumor Activity of Fused Quinoline Derivatives. III. Novel N-Glycosylaminoindolo[3,2-b]quinolines. Chem. Pharm. Bull. 1992, 40, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Goon, S.; Meth-Cohn, O. Carbenes from Vilsmeier reagents by the action of bases in POCl3; the umpolung of Vilsmeier reagents. Chem. Commun. 1996, 1395–1396. [Google Scholar] [CrossRef]
- Du, W.; Curran, D.P. Synthesis of Carbocyclic and Heterocyclic Fused Quinolines by Cascade Radical Annulations of Unsaturated N-Aryl Thiocarbamates, Thioamides, and Thioureas. Org. Lett. 2003, 5, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, P.T.; Parameswaran, P.S.; Tilve, S.G. Double reductive cyclization: A facile synthesis of the indoloquinoline alkaloid cryptotackieine. Tetrahedron Lett. 2007, 48, 7870–7872. [Google Scholar] [CrossRef]
- Schmittel, M.; Steffen, J.-P.; Rodríguez, D.; Engelen, B.; Neumann, E.; Cinar, M.E. Thermal C2–C6 Cyclization of Enyne–Carbodiimides: Experimental Evidence Contradicts a Diradical and Suggests a Carbene Intermediate. J. Org. Chem. 2008, 73, 3005–3016. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Ishida, T.; Takemoto, Y. Synthesis of Indolo[2,3-b]quinolines by Palladium-catalyzed Annulation of Unsaturated Isothioureas. Chem. Lett. 2009, 38, 772–773. [Google Scholar] [CrossRef]
- Haddadin, M.J.; El-Nachef, C.; Kisserwani, H.; Chaaban, Y.; Kurth, M.J.; Fettinger, J.C. 2-Benzyliden-2H-thieto[3,2-b]quinoline: A new heterocycle and its rearrangement to 2-phenylthieno[3,2-b]quinoline. Tetrahedron Lett. 2010, 51, 6687–6689. [Google Scholar] [CrossRef]
- Chen, K.; Tang, X.-Y.; Shi, M. Rh(II)-Catalyzed formation of pyrrolo[2,3-b]quinolines from azide-methylenecyclopropanes and isonitriles. Chem. Commun. 2016, 52, 1967–1970. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-Z.; Hu, X.-B.; Xu, Q.; Shi, M. Thermally induced formal [3+2] cyclization of ortho-aminoaryl-tethered alkylidenecyclopropanes: Facile synthesis of furoquinoline and thienoquinoline derivatives. Chem. Commun. 2016, 52, 2701–2704. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-S.; Wang, M.-X.; Xu, D.-C.; Xie, J.-W. Synthesis of 2,3-Dihydrothieno(2,3-b)quinolines and Thieno(2,3-b)quinolines via an Unexpected Domino Aza-MBH/Alkylation/Aldol Reaction. J. Org. Chem. 2016, 81, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-C.; Liu, T.; Cheng, Y.-F.; Chang, H.-H.; Li, X.; Zhou, R.; Wei, W.-L.; Qiao, Y. AlCl3-Catalyzed Intramolecular Cyclization of N-Arylpropynamides with N-Sulfanylsuccinimides: Divergent Synthesis of 3-Sulfenyl Quinolin-2-ones and Azaspiro[4,5]trienones. J. Org. Chem. 2017, 82, 13459–13467. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.; Yoshimura, Y.; Akehi, M.; Tsuchimoto, T. A Heteroarylamine Library: Indium-Catalyzed Nucleophilic Aromatic Substitution of Alkoxyheteroarenes with Amines. Adv. Synth. Catal. 2018. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Hatanaka, K.; Shirakawa, E.; Kawakami, Y. Indium triflate-catalysed double addition of heterocyclic arenes to alkynes. Chem. Commun. 2003, 2454–2455. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Matsubayashi, H.; Kaneko, M.; Nagase, Y.; Miyamura, T.; Shirakawa, E. Indium-Catalyzed Annulation of 2-Aryl- and 2-Heteroarylindoles with Propargyl Ethers: Concise Synthesis and Photophysical Properties of Diverse Aryl- and Heteroaryl-Annulated[a]carbazoles. J. Am. Chem. Soc. 2008, 130, 15823–15835. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, T.; Kanbara, M. Reductive Alkylation of Indoles with Alkynes and Hydrosilanes under Indium Catalysis. Org. Lett. 2011, 13, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, T.; Igarashi, M.; Aoki, K. Exclusive Synthesis of β-Alkylpyrroles under Indium Catalysis: Carbonyl Compounds as Sources of Alkyl Groups. Chem. Eur. J. 2010, 16, 8975–8979. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, S.; Hondo, T.; Tsuchimoto, T. Easy Access to a Library of Alkylindoles: Reductive Alkylation of Indoles with Carbonyl Compounds and Hydrosilanes under Indium Catalysis. Adv. Synth. Catal. 2016, 358, 1136–1149. [Google Scholar] [CrossRef]
- Nomiyama, S.; Ogura, T.; Ishida, H.; Aoki, K.; Tsuchimoto, T. Indium-Catalyzed Regioselective β-Alkylation of Pyrroles with Carbonyl Compounds and Hydrosilanes and Its Application to Construction of a Quaternary Carbon Center with a β-Pyrrolyl Group. J. Org. Chem. 2017, 82, 5178–5197. [Google Scholar] [CrossRef] [PubMed]
- Yanada, R.; Hashimoto, K.; Tokizane, R.; Miwa, Y.; Minami, H.; Yanada, K.; Ishikura, M.; Takemoto, Y. Indium(III)-Catalyzed Tandem Reaction with Alkynylbenzaldehydes and Alkynylanilines to Heteroaromatic Compounds. J. Org. Chem. 2008, 73, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Tsuji, H.; Yamagata, K.-I.; Endo, K.; Tanaka, I.; Nakamura, M.; Nakamura, E. Efficient Formation of Ring Structures Utilizing Multisite Activation by Indium Catalysis. J. Am. Chem. Soc. 2008, 130, 17161–17167. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yamamoto, K.; Yamanobe, A.; Ito, K.; Kinoshita, H.; Ichikawa, J.; Hosomi, A. Indium(III)-catalyzed Coupling between Alkynes and Aldehydes to α,β-Unsaturated Ketones. Chem. Lett. 2010, 39, 766–767. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Okita, A.; Yasuda, M.; Baba, A. Indium Tribromide Catalyzed Cross-Claisen Condensation between Carboxylic Acids and Ketene Silyl Acetals Using Alkoxyhydrosilanes. Angew. Chem. Int. Ed. 2011, 50, 8623–8625. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Okita, A.; Yasuda, M.; Baba, A. Synthesis of a Wide Range of Thioethers by Indium Triiodide Catalyzed Direct Coupling between Alkyl Acetates and Thiosilanes. Org. Lett. 2012, 14, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Loh, T.-P. Asymmetric Hetero-Diels–Alder Reaction of Danishefsky’s Dienes with α-Carbonyl Esters Catalyzed by an Indium(III)–PyBox Complex. Org. Lett. 2013, 15, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Nishimoto, Y.; Yasuda, M.; Baba, A. Indium Chloride Catalyzed Alkylative Rearrangement of Propargylic Acetates Using Alkyl Chlorides, Alcohols, and Acetates: Facile Synthesis of α-Alkyl-α,β-Unsaturated Carbonyl Compounds. Org. Lett. 2014, 16, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, N.; Prajapati, D. Indium-catalyzed, novel route to β,β-disubstituted indanones via tandem Nakamura addition–hydroarylation–decarboxylation sequence. Chem. Commun. 2015, 51, 3347–3350. [Google Scholar] [CrossRef] [PubMed]
- Hamachi, Y.; Katano, M.; Ogiwara, Y.; Sakai, N. Production of Quaternary α-Aminonitriles by Means of Indium-Catalyzed Three-Component Reaction of Alkynes, Amines, and Trimethylsilyl Cyanide. Org. Lett. 2016, 18, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Pathipati, S.R.; van der Werf, A.; Eriksson, L.; Selander, N. Diastereoselective Synthesis of Cyclopenta[c]furans by a Catalytic Multicomponent Reaction. Angew. Chem. Int. Ed. 2016, 55, 11863–11866. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Wang, S.-W.; Long, Y. Indium-mediated Organic Reactions and their Application in Organic Synthesis. Curr. Org. Chem. 2016, 20, 2865–2880. [Google Scholar] [CrossRef]
- For the synthesis of HA[b]Qs with one type of a heteroaryl ring, see: References 10–16, 18–21, 25–28, 30–36, 38, 40–50, 52 and 53.
- For the synthesis of HA[b]Qs with two types of heteroaryl rings, see: References 17, 22, 37, 39 and 51.
- For the synthesis of HA[b]Qs with three types of heteroaryl rings, see: References 24 and 29. The synthesis of HA[b]Qs with three types of heteroaryl rings is also achieved in references 4 and 23 but uses the same synthetic method as in reference 42 cited within reference 72.
- Ansah, C.; Mensah, K.B. A review of the anticancer potential of the antimalarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Ghana Med. J. 2013, 47, 137–147. [Google Scholar] [PubMed]
- Gibeau, A.L.; Snyder, J.K. Indium(III)-Catalyzed Hydrative Cyclization of 1,7-Diynyl Ethers. Org. Lett. 2011, 13, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, S.; Pan, Y.; Xu, Y.; Wang, H. The indium-catalysed hydration of alkynes using substoichiometric amounts of PTSA as additive. Tetrahedron 2013, 69, 3775–3781. [Google Scholar] [CrossRef]
- Avery, M.A.; Verlander, M.S.; Goodman, M. Synthesis of 6-Aminoisoproterenol. J. Org. Chem. 1980, 45, 2750–2753. [Google Scholar] [CrossRef]
- Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley-VCH: Weinheim, Germany, 2013; pp. 205–278. ISBN 978-3-527-31861-2. [Google Scholar]
- Boahen, Y.O.; Mann, J. Synthesis and evaluation of quindoline and its analogue as potential anticancer agents. Chem. Nat. Compd. 2014, 50, 494–497. [Google Scholar] [CrossRef]
- Arzel, E.; Rocca, P.; Grellier, P.; Labaeïd, M.; Frappier, F.; Guéritte, F.; Gaspard, C.; Marsais, F.; Godard, A.; Quéguiner, G. New Synthesis of Benzo-δ-carbolines, Cryptolepines, and Their Salts: In Vitro Cytotoxic, Antiplasmodial, and Antitrypanosomal Activities of δ-Carbolines, Benzo-δ-carbolines, and Cryptolepines. J. Med. Chem. 2001, 44, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.G.; Hartley, J.P.; Griffin, D. Counterion effects in indium-catalysed aromatic electrophilic substitution reactions. Tetrahedron Lett. 2002, 43, 4789–4791. [Google Scholar] [CrossRef]
- Nakamura, M.; Endo, K.; Nakamura, E. A Modular Approach to α-Arylated Carbonyl Compounds via Indium Tris(bistriflylamide)-Catalyzed Regioselective Addition of β-Ketoesters to 1,3-Diynes. Adv. Synth. Catal. 2005, 347, 1681–1686. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Matsubayashi, H.; Kaneko, M.; Shirakawa, E.; Kawakami, Y. Easy Access to Aryl- and Heteroaryl-Annulated[a]carbazoles by the Indium-Catalyzed Reaction of 2-Arylindoles with Propargyl Ethers. Angew. Chem. Int. Ed. 2005, 44, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Wang, H.-Y.; Winston-McPherson, G.N.; Geng, H.-M.J.; Guzei, I.A.; Tang, W. Copper-catalyzed tandem annulation/arylation for the synthesis of diindolylmethanes from propargylic alcohols. Chem. Commun. 2014, 50, 12293–12296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Miao, Z.; Sheng, C.; Yao, J.; Zhuang, C.; Zhang, W. An alternative route for synthesis of o-trifluoroacetylanilines as useful fluorine-containing intermediates. J. Fluorine Chem. 2010, 131, 800–804. [Google Scholar] [CrossRef]
- Gavai, A.V.; Delucca, G.V.; O’Malley, D.; Gill, P.; Quesnelle, C.A.; Fink, B.E.; Zhao, Y.; Lee, F.Y. Bis(fluoroalkyl)alkanoylamino-1,4-benzodiazepinone Compounds as Notch Inhibitors and Their Preparation. WO Patent 2014047372, 27 March 2014. [Google Scholar]
- Chabert, J.F.D.; Joucla, L.; David, E.; Lemaire, M. An efficient phosphine-free palladium coupling for the synthesis of new 2-arylbenzo[b]thiophenes. Tetrahedron 2004, 60, 3221–3230. [Google Scholar] [CrossRef]
- Ilangovan, A.; Satish, G. Direct Amidation of 2′-Aminoacetophenones Using I2-TBHP: A Unimolecular Domino Approach toward Isatin and Iodoisatin. J. Org. Chem. 2014, 79, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.; Renault, J.; van de Weghe, P. Palladium-catalyzed arylation of vinylic acetates. Phosphine ligand influenced regioselectivity. Tetrahedron Lett. 2009, 50, 6546–6548. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







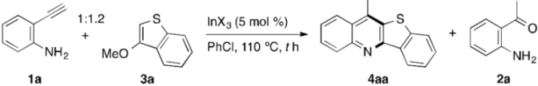
| Entry | InX3 | t (h) | Yield of 4aa (%) b | Yield of 2a (%) b |
|---|---|---|---|---|
| 1 | In(NTf2)3 | 24 | 11 | <1 |
| 2 | In(OTf)3 | 24 | 9 | <1 |
| 3 | In(ONf)3 | 24 | 14 | 2 |
| 4 | InCl3 | 24 | 2 | <1 |
| 5 | InBr3 | 24 | 3 | <1 |
| 6 | InI3 | 24 | 5 | <1 |
| 7 c | In(ONf)3 | 24 | 26 | 30 |
| 8 c | In(ONf)3 | 36 | 61 (61) d | <1 |

| Entry | Lewis Acid | Solvent | Conversion of 2a (%) b | Yield of 4aa (%) c |
|---|---|---|---|---|
| 1 | In(ONf)3 | PhCl | 86 | 62 |
| 2 | In(OTf)3 | PhCl | 73 | 55 |
| 3 | In(NTf2)3 | PhCl | 79 | 74 |
| 4 | InF3 | PhCl | 4 | <1 |
| 5 | InCl3 | PhCl | 95 | 83 |
| 6 | InBr3 | PhCl | 97 | 92 |
| 7 | InI3 | PhCl | 97 | 92 |
| 8 | In(OH)3 | PhCl | <1 | <1 |
| 9 | In(OAc)3 | PhCl | <1 | <1 |
| 10 | ScBr3 | PhCl | 61 | 50 |
| 11 | FeBr3 | PhCl | 86 | 62 |
| 12 | CoBr2 | PhCl | 31 | 22 |
| 13 | PdBr2 | PhCl | 47 | 29 |
| 14 | CuBr2 | PhCl | 28 | 18 |
| 15 | AgBr | PhCl | 3 | <1 |
| 16 | ZnBr2 | PhCl | 15 | 5 |
| 17 | PbBr2 | PhCl | <1 | <1 |
| 18 | BiBr3 | PhCl | 45 | 33 |
| 19 | None | PhCl | 5 | <1 |
| 20 | InBr3 | PhMe | 91 | 82 |
| 21 | InBr3 | Bu2O | 85 | 74 |
| 22 | InBr3 | 1,2-Diethoxyethane | 91 | 80 |
| 23 | InBr3 | 1,4-Dioxane | 87 | 66 |
| 24 | InBr3 | MeNO2 | 88 | 76 d |
| 25 | InBr3 | PrCN | 82 | 76 |
| 26 | InBr3 | BuOH | 92 | 73 |
| 27 | InBr3 | H2O | 48 | 37 |
| 28 e | InBr3 | PhCl | 89 | 70 |
| 29 f | InBr3 | PhCl | 97 | 88 |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonekura, K.; Shinoda, M.; Yonekura, Y.; Tsuchimoto, T. Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: Synthesis of Heteroaryl[b]quinolines and Subsequent Transformation to Cryptolepine Derivatives. Molecules 2018, 23, 838. https://doi.org/10.3390/molecules23040838
Yonekura K, Shinoda M, Yonekura Y, Tsuchimoto T. Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: Synthesis of Heteroaryl[b]quinolines and Subsequent Transformation to Cryptolepine Derivatives. Molecules. 2018; 23(4):838. https://doi.org/10.3390/molecules23040838
Chicago/Turabian StyleYonekura, Kyohei, Mika Shinoda, Yuko Yonekura, and Teruhisa Tsuchimoto. 2018. "Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: Synthesis of Heteroaryl[b]quinolines and Subsequent Transformation to Cryptolepine Derivatives" Molecules 23, no. 4: 838. https://doi.org/10.3390/molecules23040838