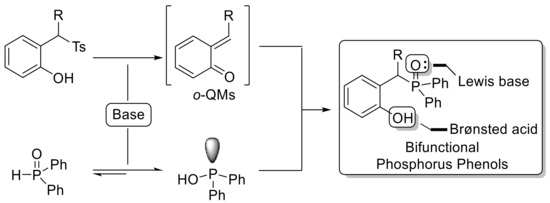
Straightforward Synthesis of Bifunctional Phosphorus Phenols via Phosphination of In Situ Generated o-Quinone Methides
Abstract
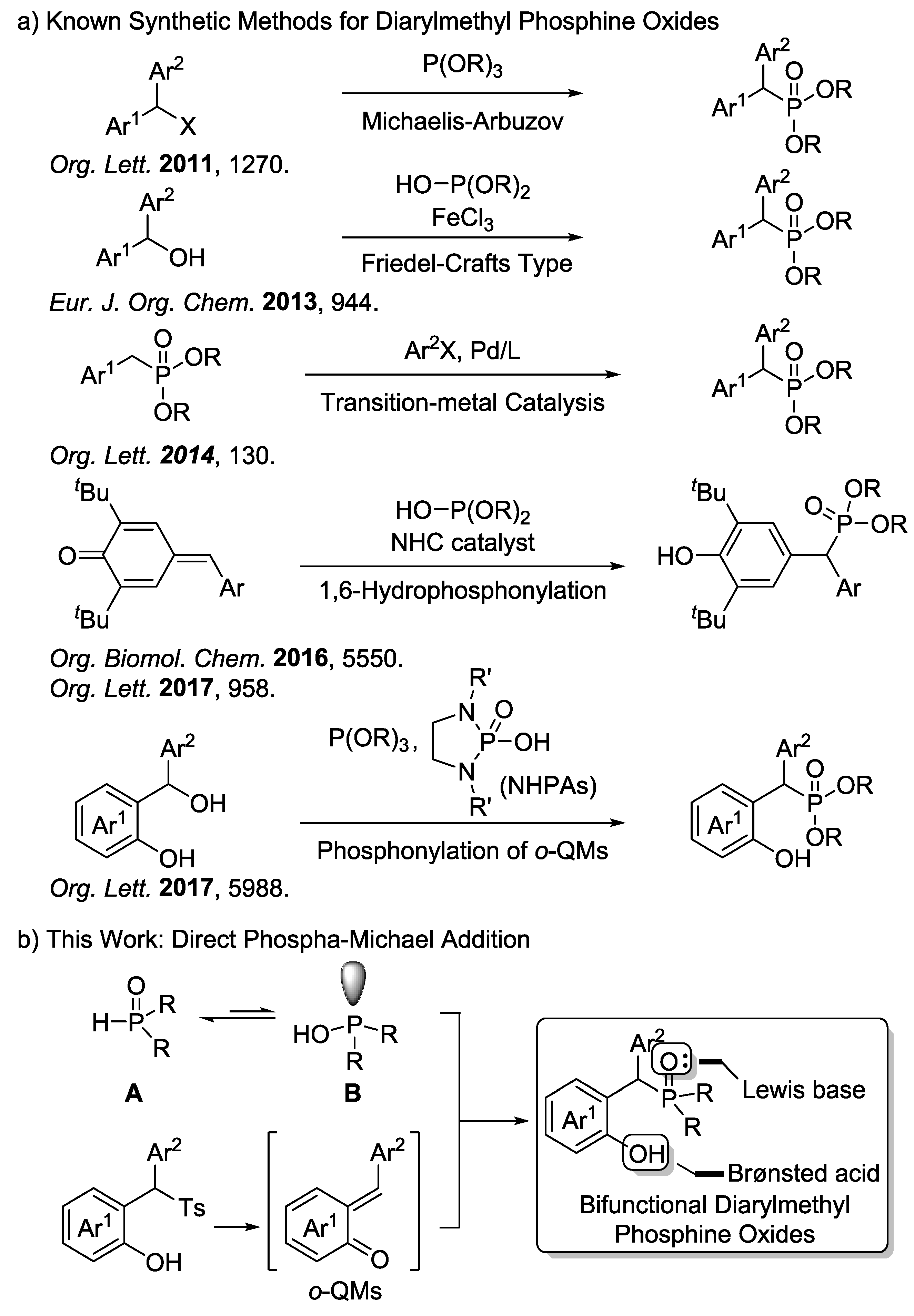
:1. Introduction
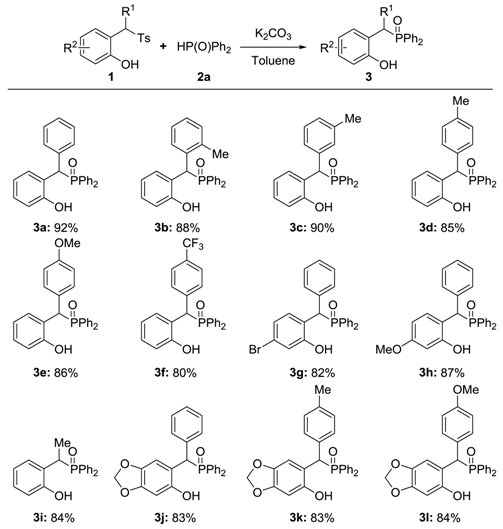
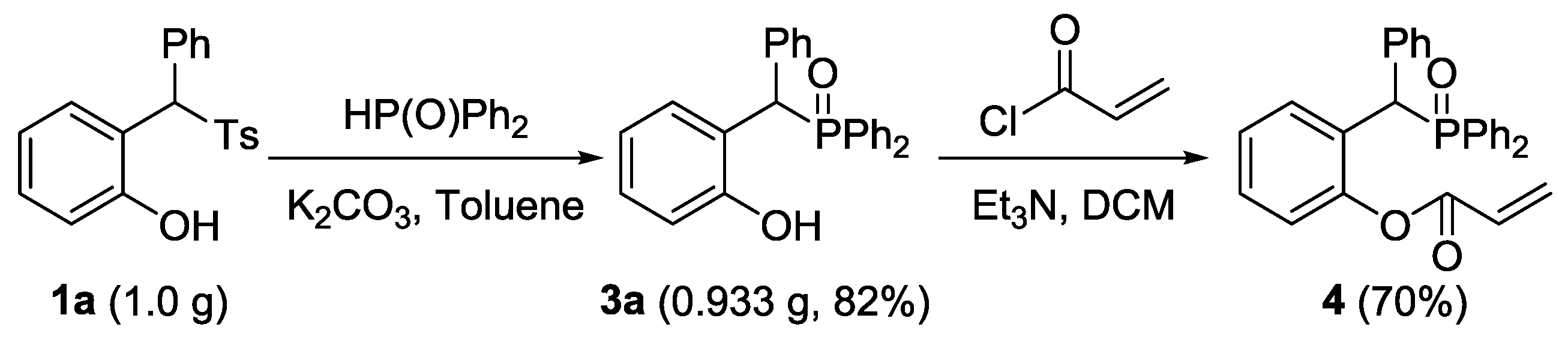
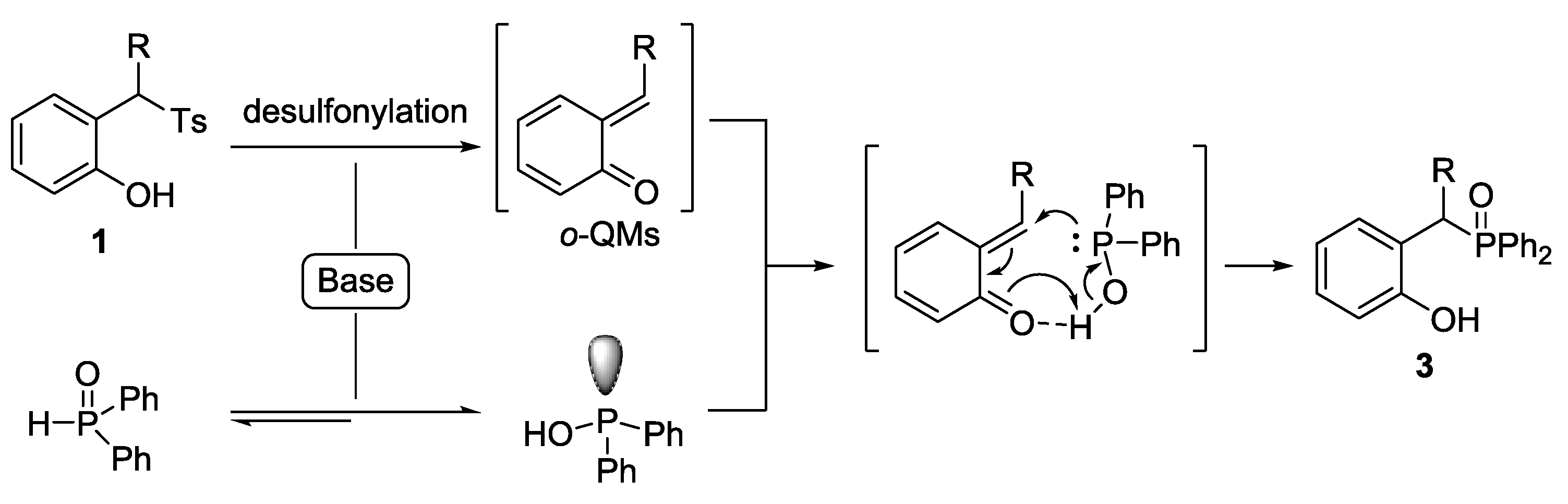
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.W.; Chin, J.P.; Quinn, J.P. Organophosphonates revealed: New insights into the microbial metabolism of ancient molecules. Nat. Rev. Microbiol. 2013, 11, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
- Grushin, V.V. Mixed Phosphine−Phosphine Oxide Ligands. Chem. Rev. 2004, 104, 1629–1662. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.L.; Kwong, F.Y.; Chan, A.S.C. Chiral Phosphorus Ligands with Interesting Properties and Practical Applications. Top. Organomet. Chem. 2011, 36, 29–66. [Google Scholar]
- Ma, J.-A. Catalytic asymmetric synthesis of α- and β-amino phosphonic acid derivatives. Chem. Soc. Rev. 2006, 35, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.J.; Fang, J.M.; Wang, S.Y.; Tsai, K.C.; Cheng, S.E.; Yang, A.S.; Hsiao, S.C.; Su, C.Y.; Wong, C.H. Synthesis of Tamiflu and its Phosphonate Congeners Possessing Potent Anti-Influenza Activity. J. Am. Chem. Soc. 2007, 129, 11892–11893. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.W. Phosphines and related P–C-bonded Compounds. Organophosphorus Chem. 2012, 41, 1–55. [Google Scholar]
- Ogawa, C.; Sugiura, M.; Kobayashi, S. Stereospecific, Enantioselective Allylation of α-Hydrazono Esters by Using Allyltrichlorosilanes with BINAP Dioxides as Neutral-Coordinate Organocatalysts. Angew. Chem. Int. Ed. 2004, 43, 6491–6493. [Google Scholar] [CrossRef] [PubMed]
- Horsman, G.P.; Zechel, D.L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef] [PubMed]
- Tokuoka, E.; Kotani, S.; Matsunaga, H.; Ishizuka, T.; Hashimoto, S.; Nakajima, M. Asymmetric ring opening of meso-epoxides catalyzed by the chiral phosphine oxide BINAPO. Tetrahedron Asymmetry 2005, 16, 2391–2392. [Google Scholar] [CrossRef]
- Jin, Y.; Du, D.M. The synthesis of phosphine oxide-linked bis(oxazoline) ligands and their application in asymmetric allylic alkylation. Tetrahedron 2012, 68, 3633–3640. [Google Scholar] [CrossRef]
- Jiang, W.-Q.; Allan, G.; Chen, X.; Fiordeliso, J.J.; Linton, O.; Tannenbaum, P.; Xu, J.; Zhu, P.-F.; Gunnet, J.; Demarest, K.; et al. Novel phosphorus-containing 17β-side chain mifepristone analogues as progesterone receptor antagonists. Steroids 2006, 71, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yamanaka, Y.; Matsushita, Y.; Fukushima, K. Flame Retardant Resin Composition and Molded Product. EP2681281A12014, 1 March 2011. [Google Scholar]
- Merino, P.; Marqués-López, E.; Herrera, R.P. Catalytic Enantioselective Hydrophosphonylation of Aldehydes and Imines. Adv. Synth. Catal. 2008, 350, 1195–1208. [Google Scholar] [CrossRef]
- Van der Jeught, S.; Stevens, C.V. Direct Phosphonylation of Aromatic Azaheterocycles. Chem. Rev. 2009, 109, 2672–2702. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, L.; Albrecht, A.; Krawczyk, H.; Jørgensen, K.A. Organocatalytic Asymmetric Synthesis of Organophosphorus Compounds. Chem. Eur. J. 2010, 16, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Demmer, C.S.; Krogsgaard-Larsen, N.; Bunch, L. Review on Modern Advances of Chemical Methods for the Introduction of a Phosphonic Acid Group. Chem. Rev. 2011, 111, 7981–8006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, R. Recent developments in metal catalyzed asymmetric addition of phosphorus nucleophiles. Chem. Soc. Rev. 2012, 41, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.K. Michaelis-Arbuzov rearrangement. Chem. Rev. 1981, 81, 415–430. [Google Scholar] [CrossRef]
- Rajeshwaran, G.G.; Nandakumar, M.; Sureshbabu, R.; Mohanakrishnan, A.K. Lewis Acid-Mediated Michaelis−Arbuzov Reaction at Room Temperature: A Facile Preparation of Arylmethyl/Heteroarylmethyl Phosphonates. Org. Lett. 2011, 13, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Pallikonda, G.; Chakravarty, M. FeCl3-Mediated Arylation of α-Hydroxyphosphonates with Unactivated Arenes: Pseudo-Umpolung in Allylic Phosphonates. Eur. J. Org. Chem. 2013, 2013, 944–951. [Google Scholar] [CrossRef]
- Montel, S.; Jia, T.; Walsh, P.J. Palladium-Catalyzed α-Arylation of Benzylic Phosphine Oxides. Org. Lett. 2014, 16, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Arde, P.; Anand, R.V. N-Heterocyclic carbene catalysed 1,6-hydrophosphonylation of p-quinone methides and fuchsones: An atom economical route to unsymmetrical diaryl- and triarylmethyl phosphonates. Org. Biomol. Chem. 2016, 14, 5550–5554. [Google Scholar] [CrossRef] [PubMed]
- Molleti, N.; Kang, J.Y. Synthesis of Diaryl Diazaphosphonates via 1,6-Hydrophosphonylation of p-Quinone Methides with N-Heterocyclic Phosphine–Thioureas. Org. Lett. 2017, 19, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kang, J.Y. Organocatalytic Phosphonylation of in situ Formed o-Quinone Methides. Org. Lett. 2017, 19, 5988–5991. [Google Scholar] [CrossRef] [PubMed]
- Chatt, J.; Heaton, B.T. The hydrolysis of monochlorophosphine and monochloroarsine complexes of platinum (II): Bridging phosphinato- and arsinato-groups. J. Chem. Soc. A 1968, 0, 2745–2757. [Google Scholar] [CrossRef]
- Ivanova, N.I.; Gusarova, N.K.; Nikitina, E.A.; Albanov, A.I.; Sinegovskaya, L.M.; Nikitin, M.V.; Konovalova, N.A.; Trofimov, B.A. Chemo- and stereoselective addition of diorganylphosphine oxides to α,β-ethylenic aldehydes. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 7–18. [Google Scholar] [CrossRef]
- Van de Water, R.W.; Pettus, T.R.R. o-Quinone methides: Intermediates underdeveloped and underutilized in organic synthesis. Tetrahedron 2002, 58, 5367–5405. [Google Scholar] [CrossRef]
- Willis, N.J.; Bray, C.D. ortho-Quinone Methides in Natural Product Synthesis. Chem. Eur. J. 2012, 18, 9160–9173. [Google Scholar] [CrossRef] [PubMed]
- Angle, S.R.; Yang, W. Synthesis and chemistry of a quinone methide model for anthracycline antitumor antibiotics. J. Am. Chem. Soc. 1990, 112, 4524–4528. [Google Scholar] [CrossRef]
- Rodriguez, R.; Moses, J.E.; Adlington, R.M.; Baldwin, J.E. A new and efficient method for o-quinone methide intermediate generation: Application to the biomimetic synthesis of the benzopyran derived natural products (±)-lucidene and (±)-alboatrin. Org. Biomol. Chem. 2005, 3, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Weinert, E.E.; Dondi, R.; Colloredo-Melz, S.; Frankenfield, K.N.; Mitchell, C.H.; Freccero, M.; Rokita, S.E. Substituents on Quinone Methides Strongly Modulate Formation and Stability of Their Nucleophilic Adducts. J. Am. Chem. Soc. 2006, 128, 11940–11947. [Google Scholar] [CrossRef] [PubMed]
- Bray, C.D. Generation and hetero-Diels–Alder reactions of an o-quinone methide under mild, anionic conditions: Rapid synthesis of mono-benzannelated spiroketals. Org. Biomol. Chem. 2008, 6, 2815–2819. [Google Scholar] [CrossRef] [PubMed]
- Bray, C.D. An Approach to Benzannelated [5,6]-Spiroketals. Synlett 2008, 2500–2502. [Google Scholar] [CrossRef]
- Arumugam, S.; Popik, V.V. Photochemical Generation and the Reactivity of o-Naphthoquinone Methides in Aqueous Solutions. J. Am. Chem. Soc. 2009, 131, 11892–11899. [Google Scholar] [CrossRef] [PubMed]
- Basarić, N.; Cindro, N.; Hou, Y.; Žabčić, I.; Mlinarić-Majerski, K.; Wan, P. Competing photodehydration and excited-state intramolecular proton transfer (ESIPT) in adamantyl derivatives of 2-phenylphenols. Can. J. Chem. 2011, 89, 221–234. [Google Scholar] [CrossRef]
- Green, J.C.; Jimnez-Alonso, S.; Brown, E.R.; Pettus, T.R.R. Total Synthesis and Repudiation of the Helianane Family. Org. Lett. 2011, 13, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- Pathak, T.P.; Sigman, M.S. Applications of ortho-Quinone Methide Intermediates in Catalysis and Asymmetric Synthesis. J. Org. Chem. 2011, 76, 9210–9215. [Google Scholar] [CrossRef] [PubMed]
- Percivalle, C.; La Rosa, A.; Verga, D.; Doria, F.; Mella, M.; Palumbo, M.; Di Antonio, M.; Freccero, M. Quinone Methide Generation via Photoinduced Electron Transfer. J. Org. Chem. 2011, 76, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Radomkit, S.; Sarnpitak, P.; Tummatorn, J.; Batsomboon, P.; Ruchirawat, S.; Ploypradith, P. Pt(IV)-catalyzed generation and [4+2]-cycloaddition reactions of o-quinone methides. Tetrahedron 2011, 67, 3904–3914. [Google Scholar] [CrossRef]
- Majumdar, N.; Korthals, K.A.; Wulff, W.D. Simultaneous Synthesis of Both Rings of Chromenes via a Benzannulation/o-Quinone Methide Formation/Electrocyclization Cascade. J. Am. Chem. Soc. 2012, 134, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-W.; Cao, L.-L.; Ye, Z.-S.; Jiang, G.-F.; Zhou, Y.-G. A mild method for generation of o-quinone methides under basic conditions. The facile synthesis of trans-2,3-dihydrobenzofurans. Chem. Commun. 2013, 49, 1660. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, X.; Chen, M.-W.; Zhou, Y.-G. A Concise Synthesis of 2-(2-Hydroxyphenyl)acetonitriles via the o-Quinone Methides Generated from 2-(1-Tosylalkyl)phenols. Chin. J. Chem. 2014, 32, 981–984. [Google Scholar] [CrossRef]
- Wu, B.; Gao, X.; Yan, Z.; Chen, M.-W.; Zhou, Y.-G. C–H Oxidation/Michael Addition/Cyclization Cascade for Enantioselective Synthesis of Functionalized 2-Amino-4H-chromenes. Org. Lett. 2015, 17, 6134–6137. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, X.; Yan, Z.; Huang, W.-X.; Zhou, Y.-G. Enantioselective synthesis of functionalized 2-amino-4H-chromenes via the o-quinone methides generated from 2-(1-tosylalkyl)phenols. Tetrahedron Lett. 2015, 56, 4334–4338. [Google Scholar] [CrossRef]
- Guo, W.; Wu, B.; Zhou, X.; Chen, P.; Wang, X.; Zhou, Y.-G.; Liu, Y.; Li, C. Formal Asymmetric Catalytic Thiolation with a Bifunctional Catalyst at a Water–Oil Interface: Synthesis of Benzyl Thiols. Angew. Chem. Int. Ed. 2015, 54, 4522–4526. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gao, X.; Chen, M.-W.; Zhou, Y.-G. Direct amination of 2-(1-tosylalkyl)phenols with aqueous ammonia: A metal-free synthesis of primary amines. Tetrahedron Lett. 2015, 56, 1135–1137. [Google Scholar] [CrossRef]
- Wu, B.; Yu, Z.; Gao, X.; Lan, Y.; Zhou, Y.-G. Regioselective α-Addition of Deconjugated Butenolides: Enantioselective Synthesis of Dihydrocoumarins. Angew. Chem. Int. Ed. 2017, 56, 4006–4010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, M.-L.; Gao, X.; Jiang, G.-F.; Zhou, Y.-G. Bifunctional squaramide-catalyzed synthesis of chiral dihydrocoumarins via ortho-quinone methides generated from 2-(1-tosylalkyl)phenols. Chem. Commun. 2017, 53, 3531. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Hirotsu, K.; Yamada, S. Phosphorus-Containing Curable Resin Compositions and Cured Products Thereof with Excellent Fire and Hydrolysis Resistance. JP2013095818, 20 May 2013. [Google Scholar]
- Nakamoto, Y.; Hirotsu, K.; Yamada, S. Diarylphosphine Oxides Bearing Oxyphenyl Groups, Diarylphosphoryl Alcohols as Their Intermediates, Manufacture of Them and Intermediates, Fireproofing Agents Containing Them, and Monomers for Fire-Resistant Resins. WO2012039473, 29 March 2012. [Google Scholar]
Sample Availability: Samples of the compounds 3a–j and 4 are available from the authors. |

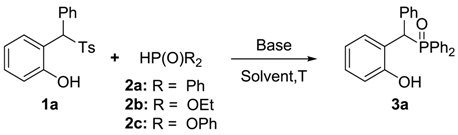
| Entry | Solvent | Base | T | Yield b/% |
|---|---|---|---|---|
| 1 | EtOAc | K2CO3 | 60 °C | 32 |
| 2 | DCE | K2CO3 | 60 °C | N.A. |
| 3 | THF | K2CO3 | 60 °C | N.A. |
| 4 | Toluene | K2CO3 | 60 °C | 36 |
| 5 | Toluene | K2CO3 | 80 °C | 73 |
| 6 | Toluene | K2CO3 | 110 °C | 92 |
| 7 | Toluene | Cs2CO3 | 110 °C | 82 |
| 8 | Toluene | Na2CO3 | 110 °C | 68 |
| 9 | Toluene | NaHCO3 | 110 °C | N.A. |
| 10 | Toluene | NaOH | 110 °C | 86 |
| 11 c | Toluene | K2CO3 | 110 °C | N.A. |
| 12 d | Toluene | K2CO3 | 110 °C | N.A. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shi, Q.; Wang, G.; Chen, S.; Hu, J. Straightforward Synthesis of Bifunctional Phosphorus Phenols via Phosphination of In Situ Generated o-Quinone Methides. Molecules 2018, 23, 1240. https://doi.org/10.3390/molecules23061240
Chen Z, Shi Q, Wang G, Chen S, Hu J. Straightforward Synthesis of Bifunctional Phosphorus Phenols via Phosphination of In Situ Generated o-Quinone Methides. Molecules. 2018; 23(6):1240. https://doi.org/10.3390/molecules23061240
Chicago/Turabian StyleChen, Zhangpei, Qinglong Shi, Gongshu Wang, Siwen Chen, and Jianshe Hu. 2018. "Straightforward Synthesis of Bifunctional Phosphorus Phenols via Phosphination of In Situ Generated o-Quinone Methides" Molecules 23, no. 6: 1240. https://doi.org/10.3390/molecules23061240
APA StyleChen, Z., Shi, Q., Wang, G., Chen, S., & Hu, J. (2018). Straightforward Synthesis of Bifunctional Phosphorus Phenols via Phosphination of In Situ Generated o-Quinone Methides. Molecules, 23(6), 1240. https://doi.org/10.3390/molecules23061240