Ball Milling Promoted N-Heterocycles Synthesis
Abstract
:1. Introduction
- Metal-catalyzed organic reactions
- nucleophilic reactions
- cascade reactions
- Diels–Alder reactions
- Oxidation-reduction reactions
- Halogenation and aminohalogenation reactions
- Formation of calixarenes, rotaxanes and cage compounds
- Transformation of biologically active compounds
- Asymmetric synthesis
2. Five Membered Rings
2.1. Pyrrole Synthesis
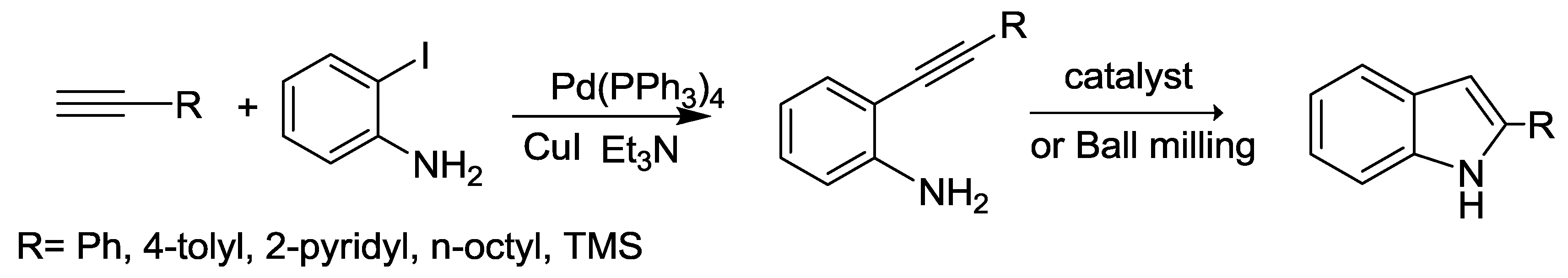
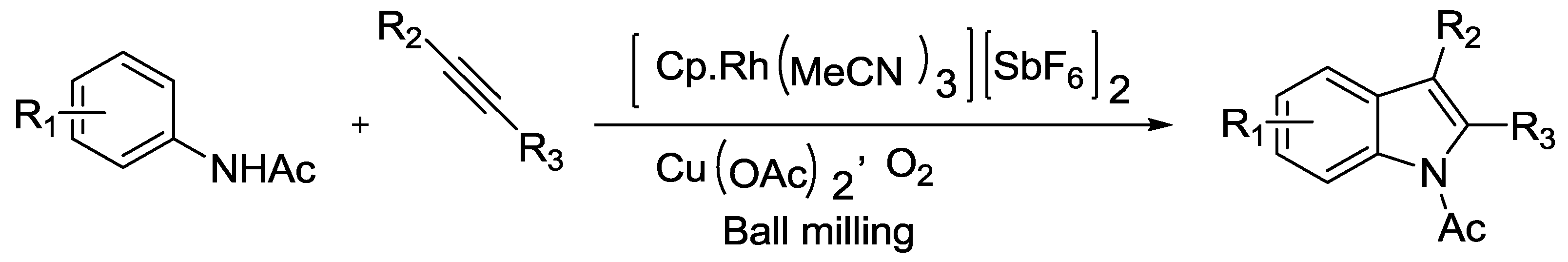
2.2. Indole Synthesis.
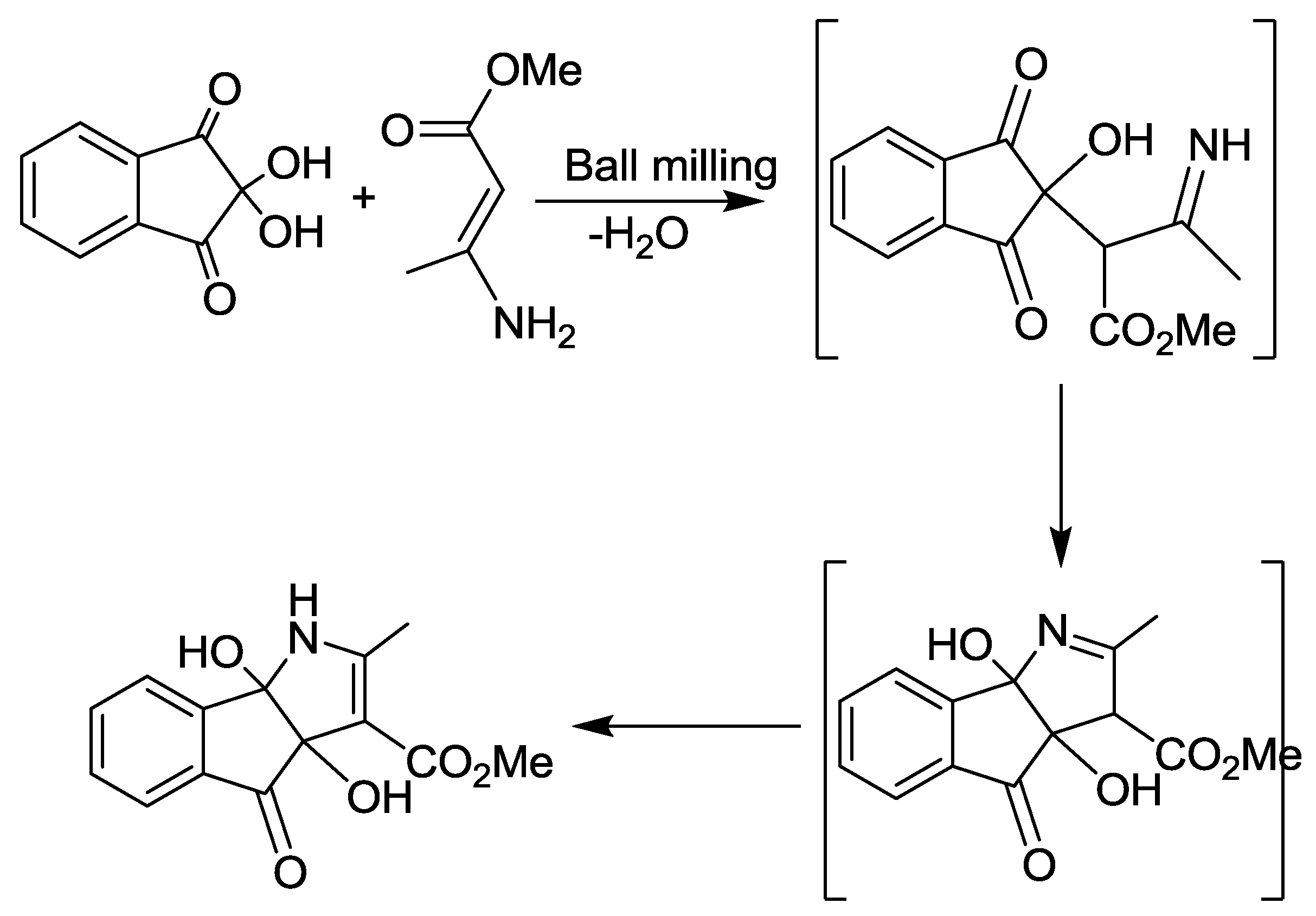
2.3. Indeno[1,2-b]pyrrole Synthesis
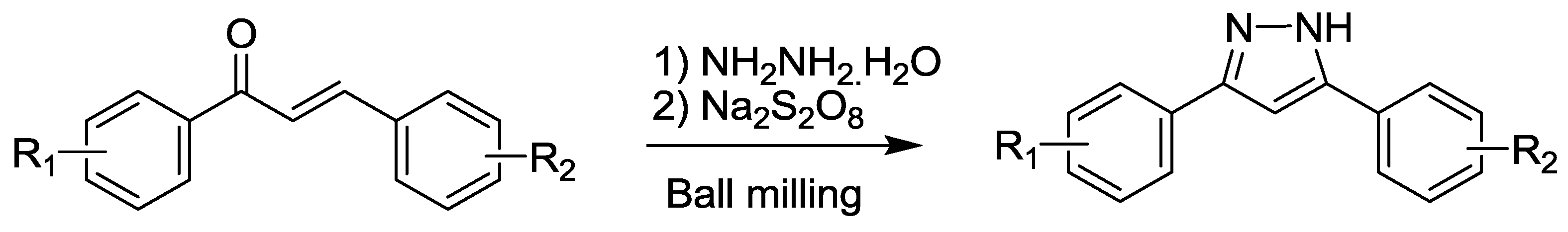
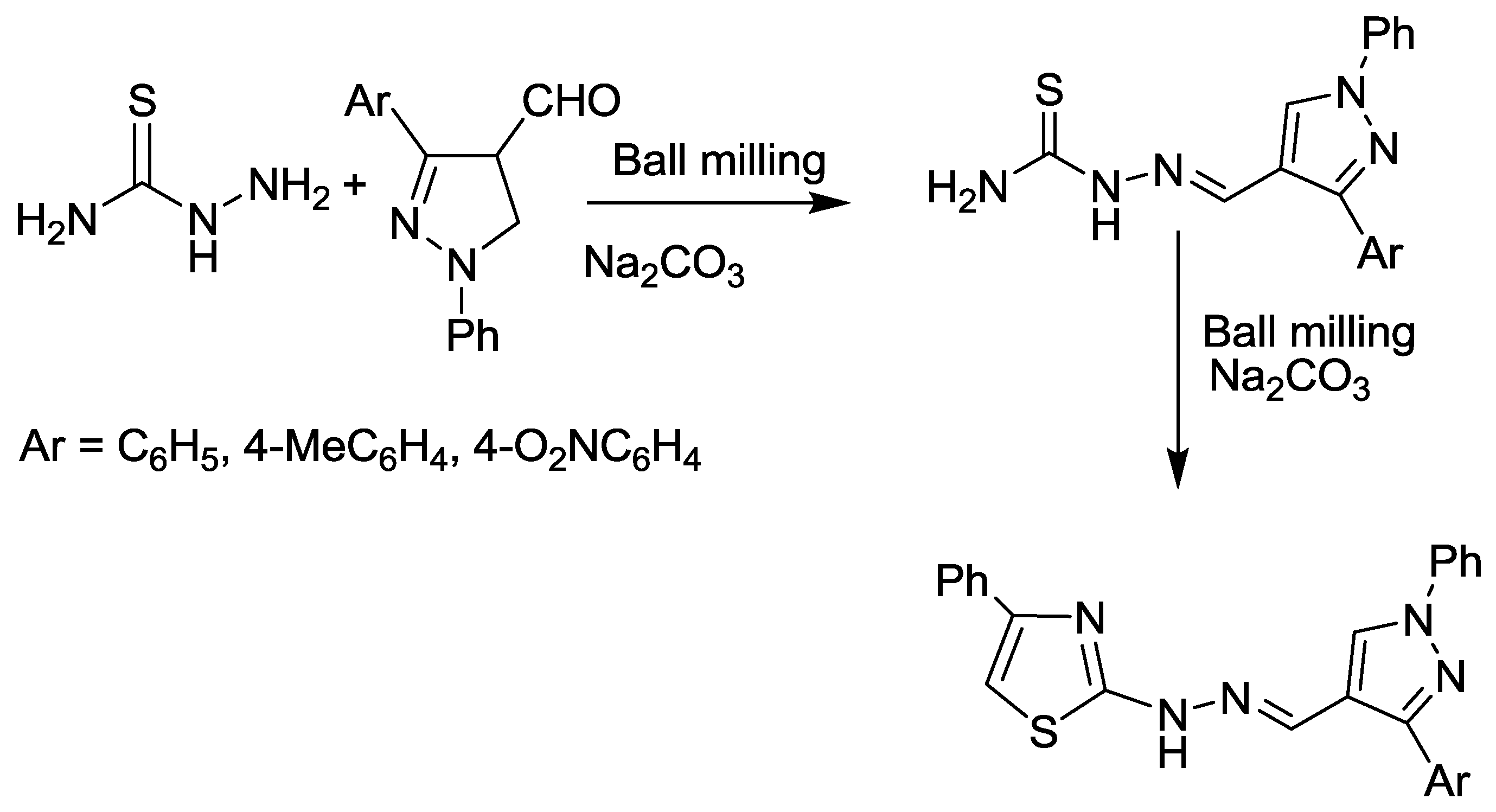
2.4. Pyrazole Synthesis
2.5. Imidazole Synthesis
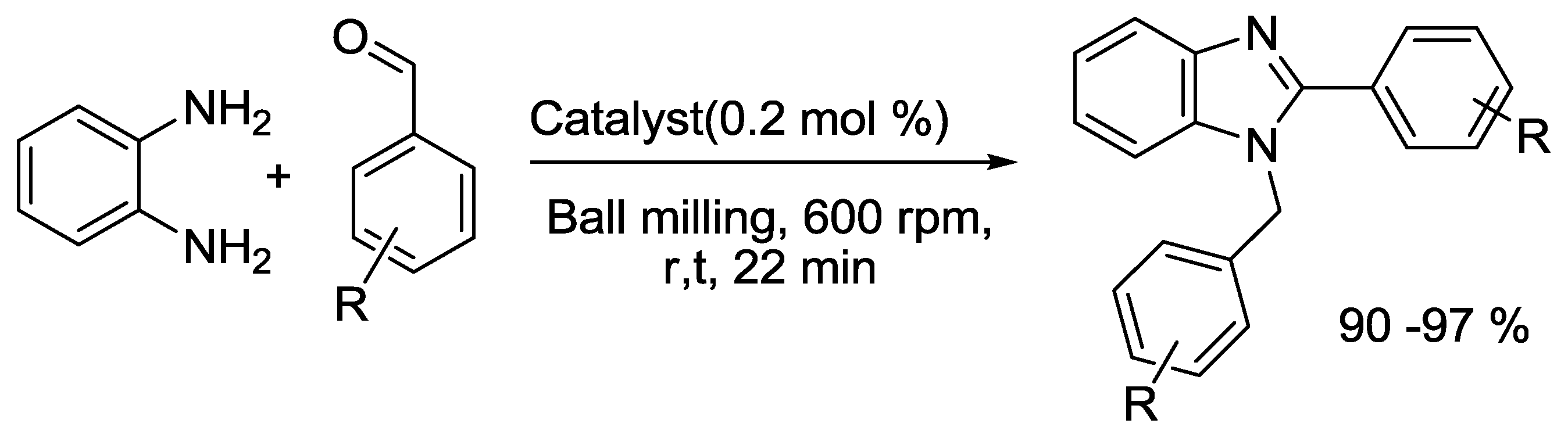
2.6. Benzimidazole Synthesis
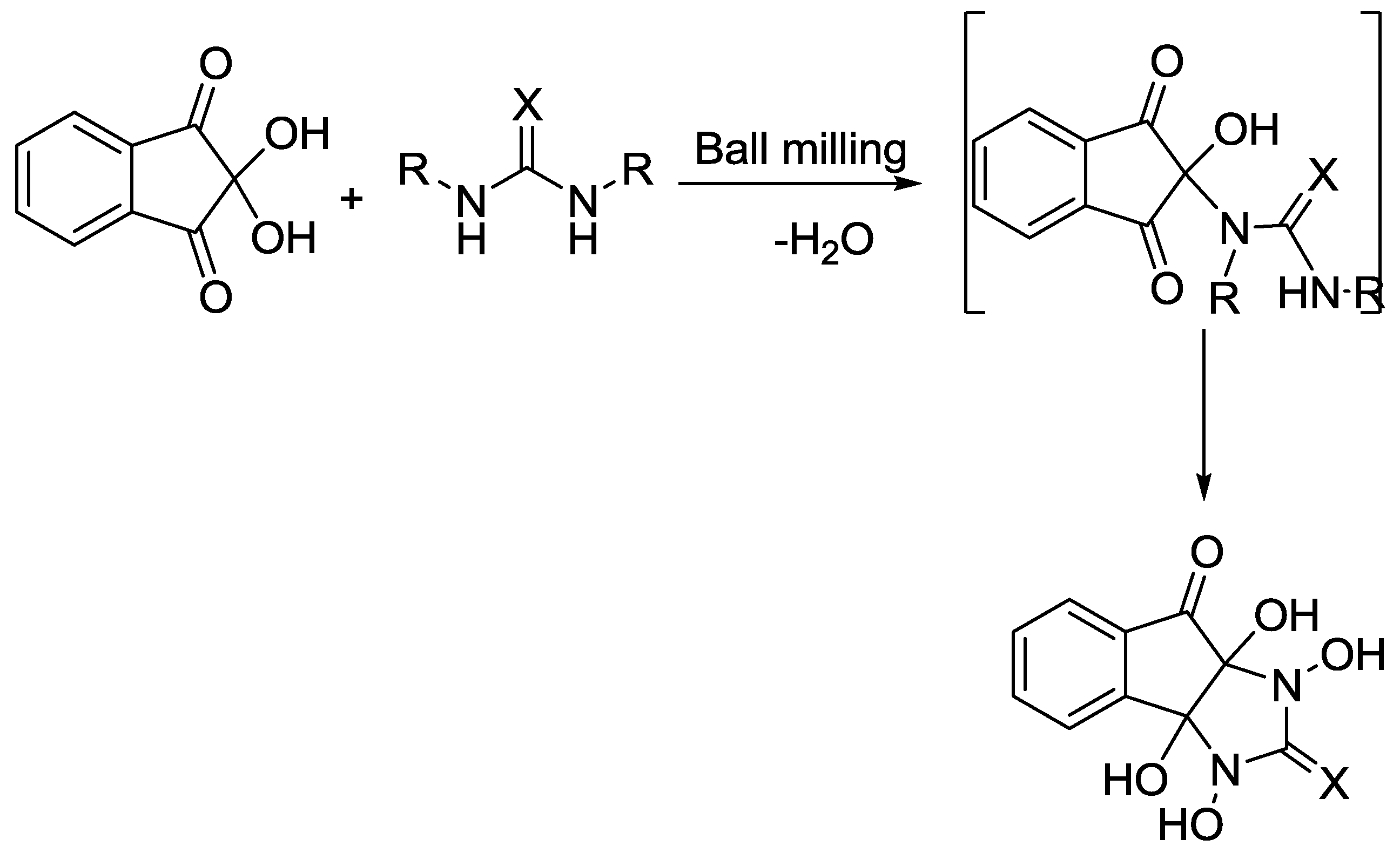
2.7. Indeno[2,1-d]imidazole Synthesis
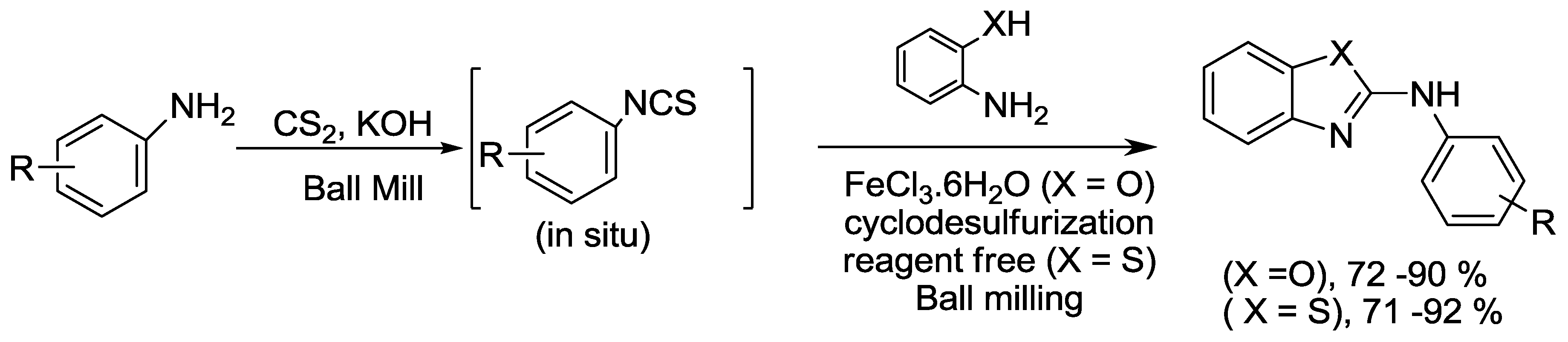
2.8. Thiazole and Oxazole Synthesis
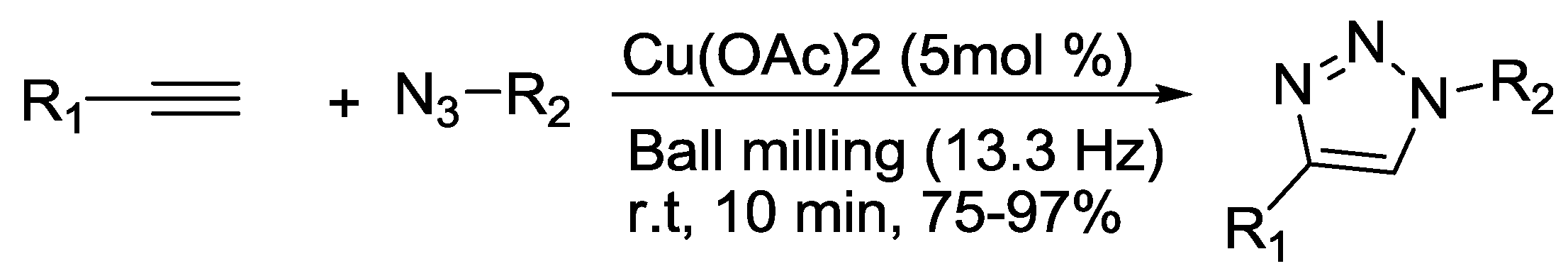
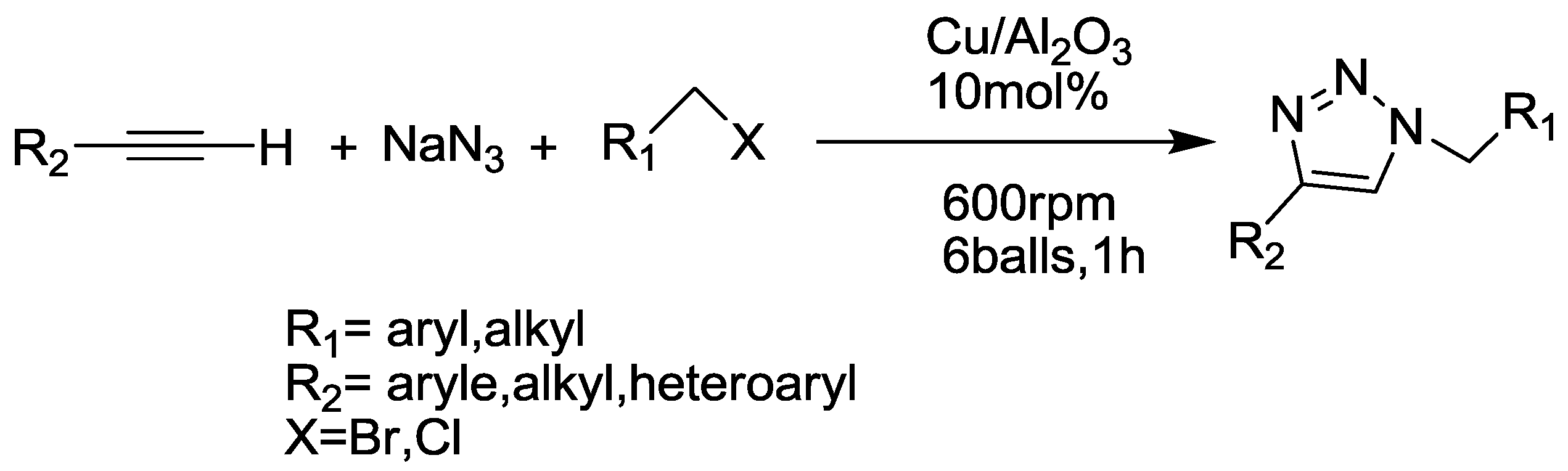
2.9. Triazole Synthesis
3. Six Membered Rings
3.1. Pyridine Synthesis
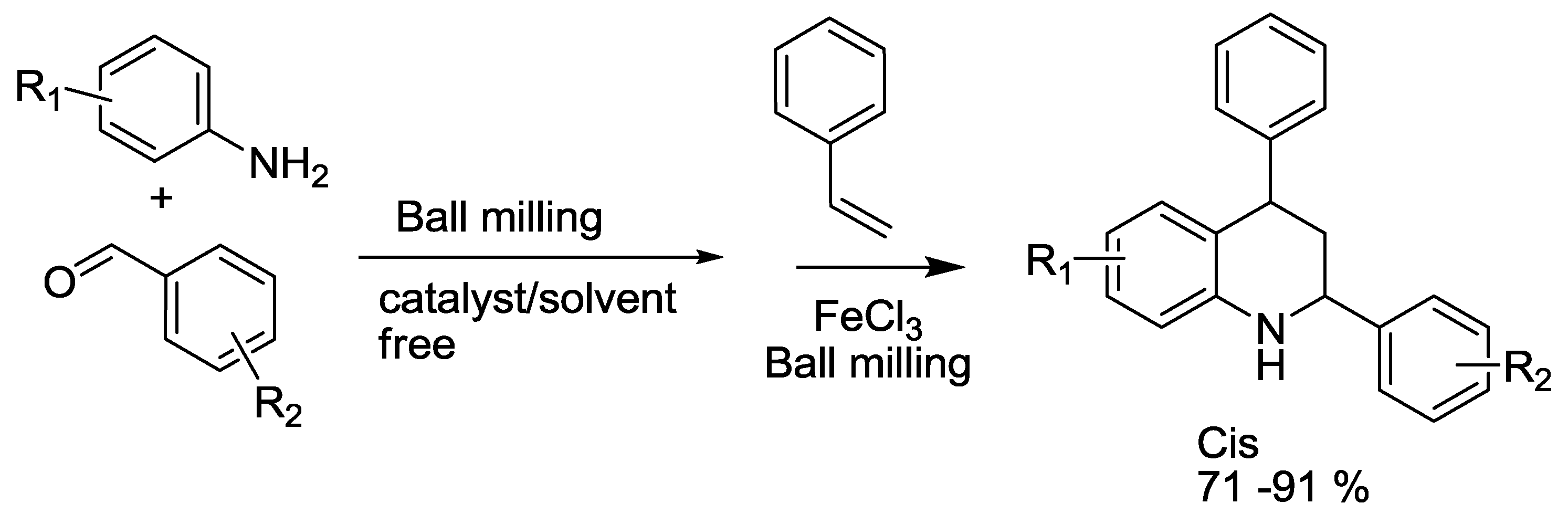
3.2. Quinoline Synthesis
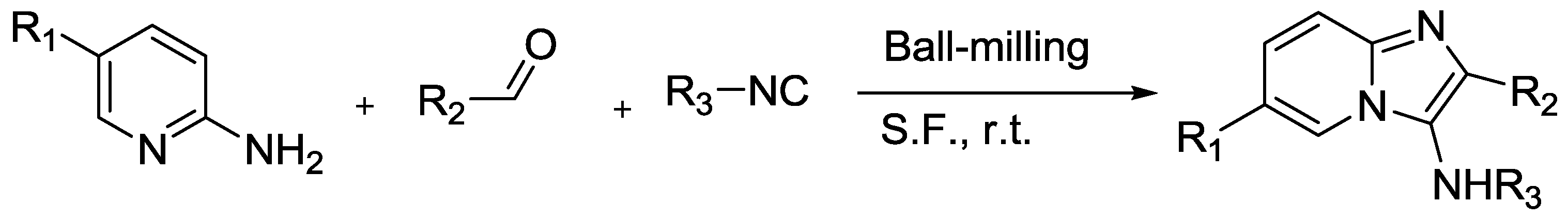
3.3. Imidazo[1,2-a]pyridine Synthesis
3.4. Chromeno[3,4-b]pyridine Synthesis
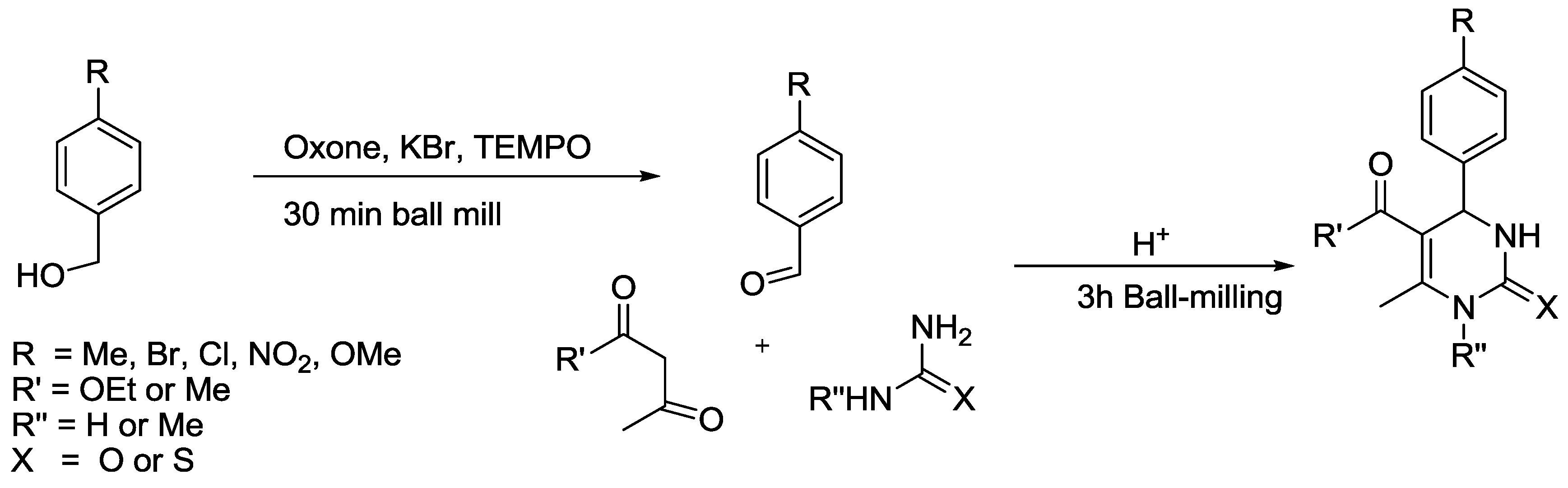
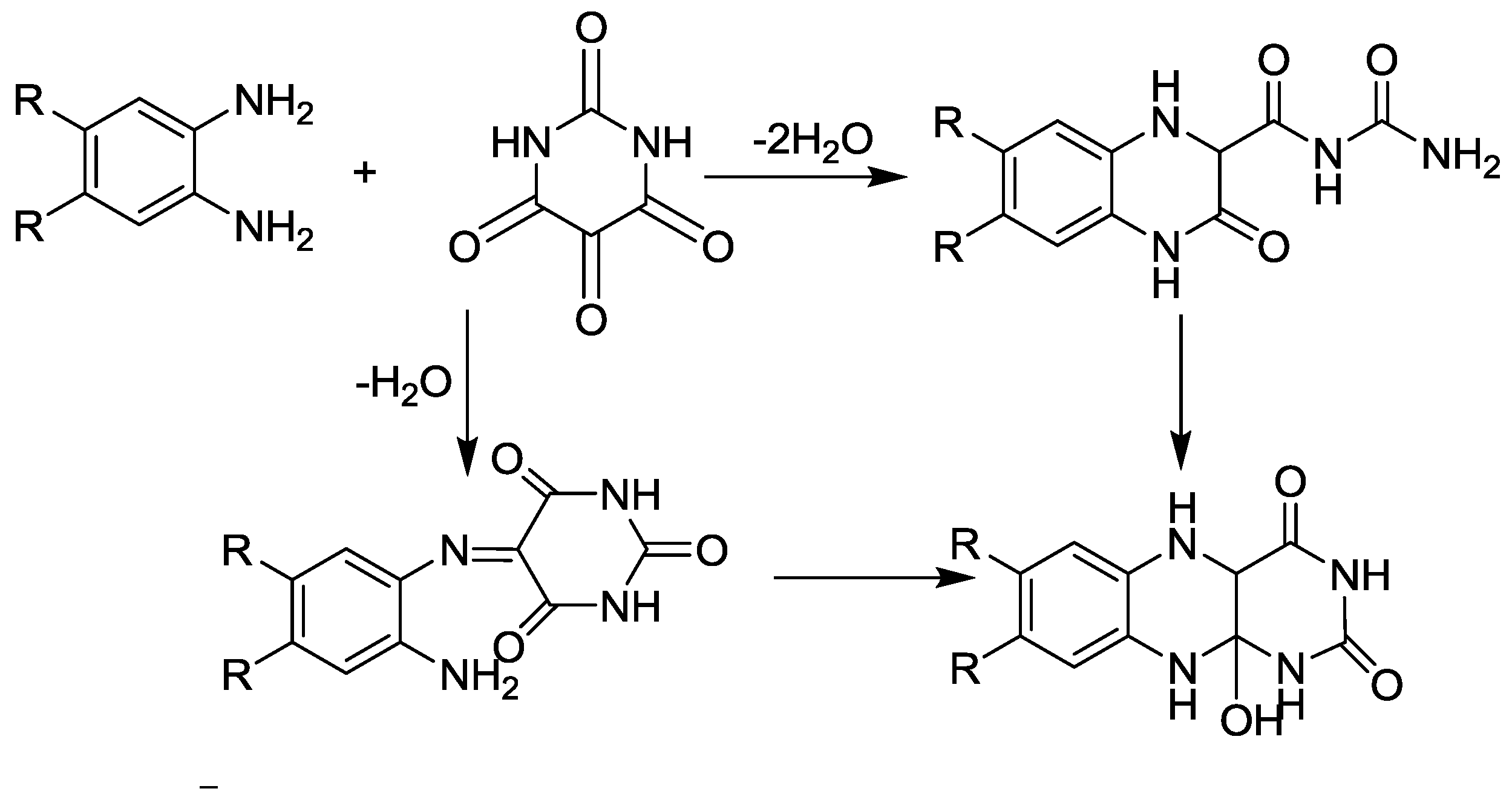
3.5. Pyrimidine Synthesis
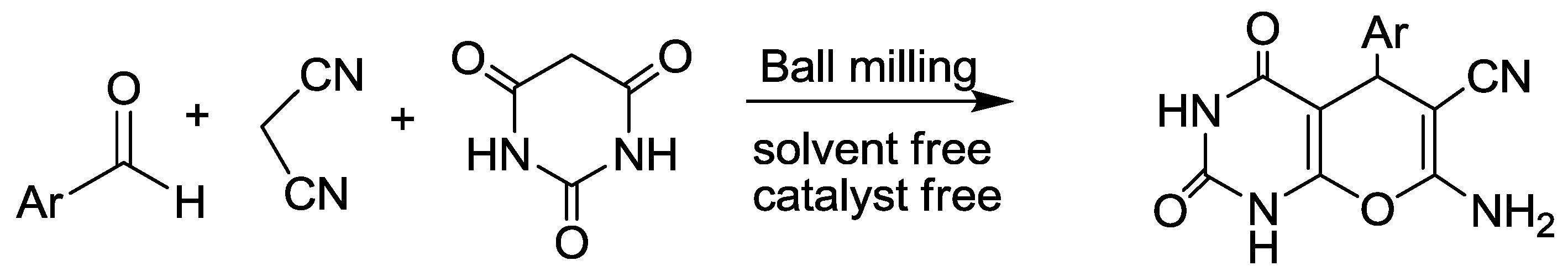
3.6. Pyrano[2,3-d]pyrimidine Synthesis
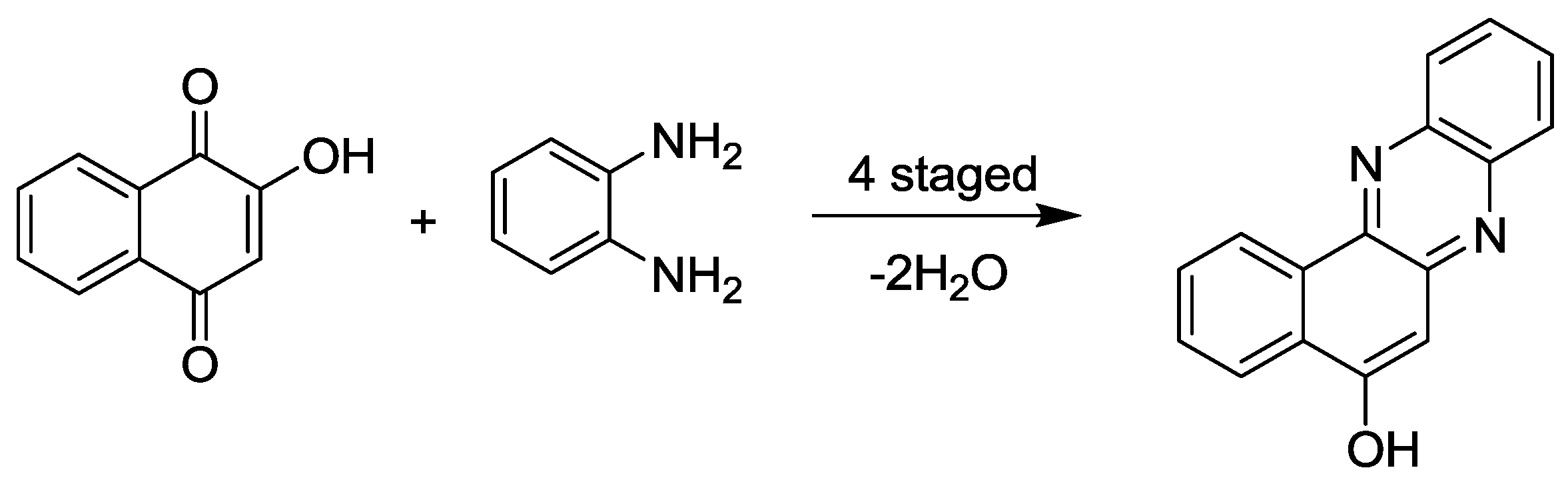
3.7. Diazine and Diazepine Synthesis
3.8. Thiazine Synthesis
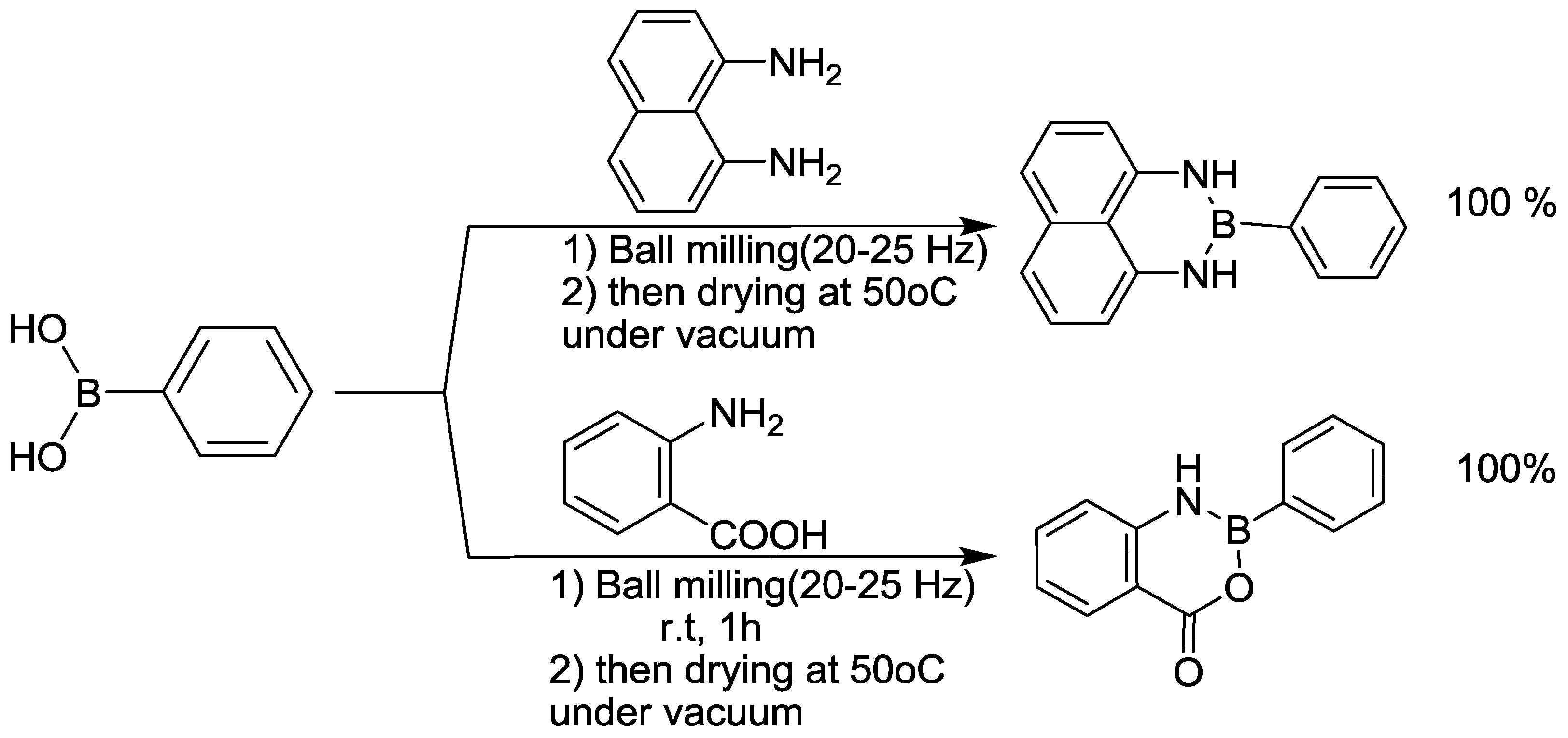
3.9. Azaborinine Synthesis
4. Higher Membered Heterocycles
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Knölker, H.-J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428. [Google Scholar] [CrossRef] [PubMed]
- Lehuédé, J.; Fauconneau, B.; Barrier, L.; Ourakow, M.; Piriou, A.; Vierfond, J.-M. Synthesis and antioxidant activity of new tetraarylpyrroles. Eur. J. Med. Chem. 1999, 34, 991–996. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.; Haas, O. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207–282. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G. Handbook of Pharmaceutical Natural Products; Wiley-VCH: Weinheim, Germany, 2010; Volume 1. [Google Scholar]
- Brahmachari, G. Green Synthetic Approaches for Biologically Relevant Heterocycles: An Overview. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G., Ed.; Elsevier: Boston, MI, USA, 2015; pp. 1–6. [Google Scholar]
- Wu, J.Y.-C.; Fong, W.-F.; Zhang, J.-X.; Leung, C.-H.; Kwong, H.-L.; Yang, M.-S.; Li, D.; Cheung, H.-Y. Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix Peucedani. Eur. J. Pharmacol. 2003, 473, 9–17. [Google Scholar] [CrossRef]
- Raj, T.; Bhatia, R.K.; Sharma, M.; Saxena, A.; Ishar, M. Cytotoxic activity of 3-(5-phenyl-3H-[1,2,4] dithiazol-3-yl) chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides. Eur. J. Med. Chem. 2010, 45, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Sugiono, E.; Merino, E. Asymmetric organocatalysis: An efficient enantioselective access to benzopyranes and chromenes. Chemistry 2008, 14, 6329–6332. [Google Scholar] [CrossRef] [PubMed]
- De Andrade-Neto, V.F.; Goulart, M.O.; da Silva Filho, J.F.; da Silva, M.J.; Pinto Mdo, C.; Pinto, A.V.; Zalis, M.G.; Carvalho, L.H.; Krettli, A.U. Antimalarial activity of phenazines from lapachol, beta-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorgan. Med. Chem. Lett. 2004, 14, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Kim, K.-C.; Jin, C.-Y.; Han, M.-H.; Park, C.; Lee, K.-J.; Park, Y.-M.; Choi, Y.H.; Kim, G.-Y. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int. Immunopharmacol. 2007, 7, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.R.; Jursic, B.S.; Hooper, C.L.; Neumann, D.M.; Thangaraj, K.; LeBlanc, B. Anticancer activity for 4,4′-dihydroxybenzophenone-2,4-dinitrophenylhydrazone (A-007) analogues and their abilities to interact with lymphoendothelial cell surface markers. Bioorg. Med. Chem. Lett. 2002, 12, 3407–3411. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A.; Sharma, S.; Ahmad, P.; Singh, A.; Bhatia, G.; Srivastava, A.K. Pyranocoumarins: A new class of anti-hyperglycemic and anti-dyslipidemic agents. Bioorgan. Med. Chem. Lett. 2009, 19, 6447–6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borad, M.A.; Bhoi, M.N.; Prajapati, N.P.; Patel, H.D. Review of Synthesis of Multispiro Heterocyclic Compounds from Isatin. Synth. Commun. 2014, 44, 1043–1057. [Google Scholar] [CrossRef]
- Sakhuja, R.; Panda, S.S.; Bajaj, K. Microwave Assisted Synthesis of Five Membered Azaheterocyclic Systems. Curr. Org. Chem. 2012, 16, 789–828. [Google Scholar]
- Andrei, Y. Introduction: Small Heterocycles in Synthesis. Chem. Rev. 2014, 114, 7783. [Google Scholar] [CrossRef] [PubMed]
- Bürli, R.W.; McMinn, D.; Kaizerman, J.A.; Hu, W.; Ge, Y.; Pack, Q.; Jiang, V.; Gross, M.; Garcia, M.; Tanaka, R. DNA binding ligands targeting drug-resistant Gram-positive bacteria. Part. 1: Internal benzimidazole derivatives. Bioorgan. Med. Chem. Lett. 2004, 14, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G.; Banerjee, B. Facile and One-Pot Access of 3,3-Bis (indol-3-yl) indolin-2-ones and 2,2-Bis (indol-3-yl) acenaphthylen-1 (2H)-one Derivatives via an Eco-Friendly Pseudo-Multicomponent Reaction at Room Temperature Using Sulfamic Acid as an Organo-Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2802–2812. [Google Scholar] [CrossRef]
- Foye, W. Principal di Chemico Farmaceutica; Piccin: Padova, Italy, 1991; p. 416. [Google Scholar]
- Kaupp, G.; Naimi-Jamal, M.; Ren, H.; Zoz, H. In Advanced Technologies Based on Self-Propogating and Mechanochemical Reactions for Environmental Protection; Cao, G., Delogu, F., Orru, R., Eds.; Research Signpost: Kerala, India, 2003; Volume 4, pp. 83–100. [Google Scholar]
- Mashkouri, S.; Naimi-Jamal, M.R. Mechanochemical solvent-free and catalyst-free one-pot synthesis of pyrano [2,3-d] pyrimidine-2,4 (1H,3H)-diones with quantitative yields. Molecules 2009, 14, 474–479. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; IUPAC Recommendations; IUPAC: Zürich, Switzerland, 1997. [Google Scholar]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The mechanical activation of covalent bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef] [PubMed]
- Todres, Z.V. Organic Mechanochemistry and Its Practical Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177. [Google Scholar] [CrossRef]
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Ould M’hamed, M. Ball Milling for Heterocyclic Compounds Synthesis in Green Chemistry: A Review. Synth. Commun. 2015, 45, 2511–2528. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Margetic, D.; Štrukil, V. Mechanochemical Organic Synthesis; Elsevier: New York, NY, USA, 2016. [Google Scholar]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-C.; Xu, H.; Yu, F.; Zhang, Z. Manganese (III) acetate mediated synthesis of polysubstituted pyrroles under solvent-free ball milling. Tetrahedron Lett. 2017, 58, 674–678. [Google Scholar] [CrossRef]
- Kaupp, G.; Schmeyers, J.; Kuse, A.; Atfeh, A. Cascade Reactions in Quantitative Solid-State Syntheses. Angew. Chem. Int. Ed. 1999, 38, 2896–2899. [Google Scholar] [CrossRef]
- Zille, M.; Stolle, A.; Wild, A.; Schubert, U.S. ZnBr 2-mediated synthesis of indoles in a ball mill by intramolecular hydroamination of 2-alkynylanilines. RSC Adv. 2014, 4, 13126–13133. [Google Scholar] [CrossRef]
- Hermann, G.N.; Jung, C.L.; Bolm, C. Mechanochemical indole synthesis by rhodium-catalysed oxidative coupling of acetanilides and alkynes under solventless conditions in a ball mill. Green Chem. 2017, 19, 2520–2523. [Google Scholar] [CrossRef]
- Jerezano, A.V.; Labarrios, E.M.; Jiménez, F.E.; del Carmen Cruz, M.; Pazos, D.C.; Gutiérrez, R.U.; Delgado, F.; Tamariz, J. Iodine-mediated one-pot synthesis of indoles and 3-dimethylaminoindoles via annulation of enaminones. Arkivoc 2014, 3, 18–53. [Google Scholar]
- Vadivelu, M.; Sugirdha, S.; Dheenkumar, P.; Arun, Y.; Karthikeyan, K.; Praveen, C. Solvent-free implementation of two dissimilar reactions using recyclable CuO nanoparticles under ball-milling conditions: Synthesis of new oxindole-triazole pharmacophores. Green Chem. 2017, 19, 3601–3610. [Google Scholar] [CrossRef]
- Kaupp, G.; Naimi-Jamal, M.R. Quantitative cascade condensations between o-phenylenediamines and 1, 2-dicarbonyl compounds without production of wastes. Eur. J. Org. Chem. 2002, 2002, 1368–1373. [Google Scholar] [CrossRef]
- Paveglio, G.C.; Longhi, K.; Moreira, D.N.; München, T.S.; Tier, A.Z.; Gindri, I.M.; Bender, C.R.; Frizzo, C.P.; Zanatta, N.; Bonacorso, H.G. How Mechanical and Chemical Features Affect the Green Synthesis of 1 H-Pyrazoles in a Ball Mill. ACS Sustain. Chem. Eng. 2014, 2, 1895–1901. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Z.; Jin, C.; Xu, L.; Wu, Q.; Su, W. Mechanically activated synthesis of 1,3, 5-triaryl-2-pyrazolines by high speed ball milling. Green Chem. 2009, 11, 163–165. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Y.-J.; Wang, C.-S. One-pot synthesis of 3,5-diphenyl-1H-pyrazoles from chalcones and hydrazine under mechanochemical ball milling. Heterocycles 2014, 89, 103–112. [Google Scholar] [CrossRef]
- Howard, J.L.; Nicholson, W.; Sagatov, Y.; Browne, D.L. One-pot multistep mechanochemical synthesis of fluorinated pyrazolones. Beilstein J. Org. Chem. 2017, 13, 1950–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondock, S.; El-Azap, H.; Kandeel, E.-E.M.; Metwally, M.A. Eco-friendly solvent-free synthesis of thiazolylpyrazole derivatives. Monatshefte Chem. Chem. Mon. 2008, 139, 1329–1335. [Google Scholar] [CrossRef]
- Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. A more sustainable and efficient access to IMes·HCl and IPr·HCl by ball-milling. Green Chem. 2018, 20, 964–968. [Google Scholar] [CrossRef]
- Sharma, H.; Kaur, N.; Singh, N.; Jang, D.O. Synergetic catalytic effect of ionic liquids and ZnO nanoparticles on the selective synthesis of 1,2-disubstituted benzimidazoles using a ball-milling technique. Green Chem. 2015, 17, 4263–4270. [Google Scholar] [CrossRef]
- EL-Sayed, T.H.; Aboelnaga, A.; Hagar, M. Ball Milling Assisted Solvent and Catalyst Free Synthesis of Benzimidazoles and Their Derivatives. Molecules 2016, 21, 1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.-J.; Wu, H.-H.; Tan, Y.-J. Straightforward synthesis of 2-anilinobenzoxazoles/benzothiazoles via mechanochemical ball-milling promoted one-pot reactions. Chem. Lett. 2015, 44, 440–441. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Metwally, M.A. Waste-Free Solid-state organic syntheses: Solvent-free alkylation, heterocyclization, and azo-coupling reactions. Monatshefte Chem. Chem. Mon. 2007, 138, 771–776. [Google Scholar] [CrossRef]
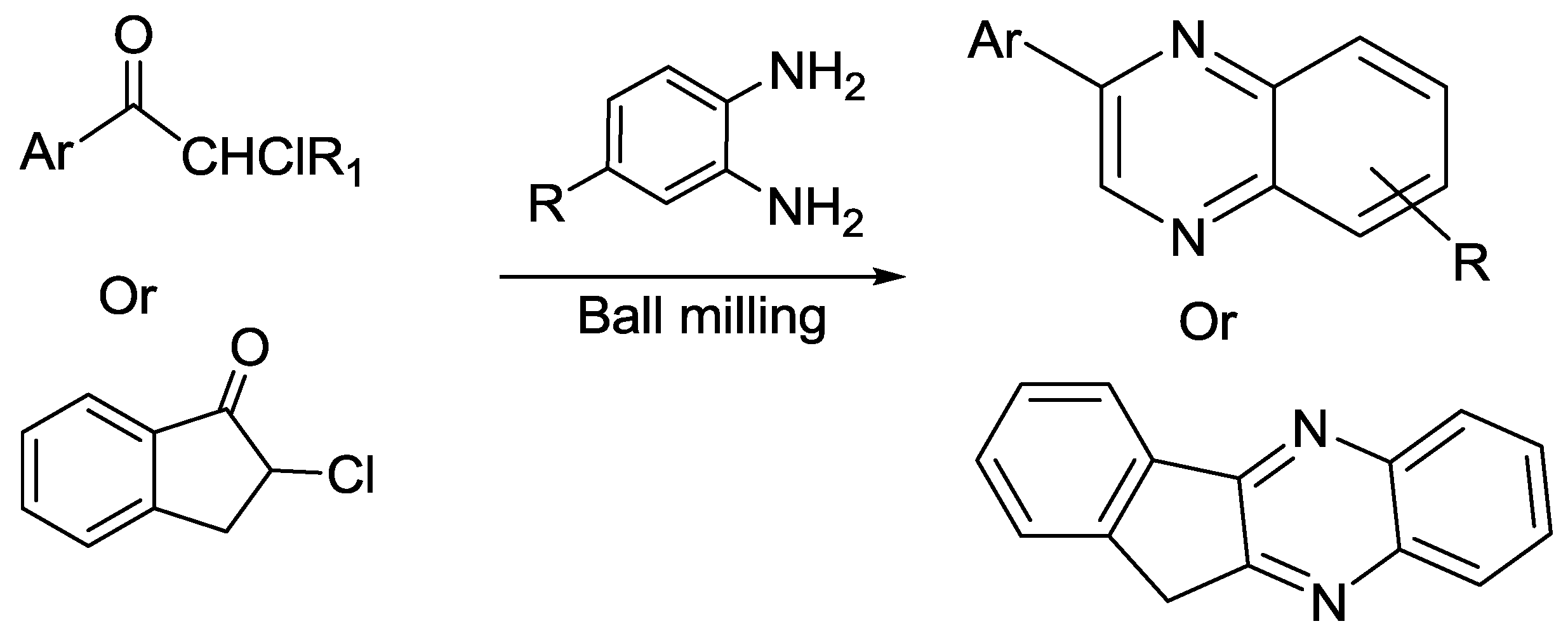
- Nagarajaiah, H.; Mishra, A.K.; Moorthy, J.N. Mechanochemical solid-state synthesis of 2-aminothiazoles, quinoxalines and benzoylbenzofurans from ketones by one-pot sequential acid-and base-mediated reactions. Org. Biomol. Chem. 2016, 14, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Thorwirth, R.; Stolle, A.; Ondruschka, B.; Wild, A.; Schubert, U.S. Fast, ligand-and solvent-free copper-catalyzed click reactions in a ball mill. Chem. Commun. 2011, 47, 4370–4372. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Ahammed, S.; Bhadra, S.; Ranu, B.C. Solvent-free one-pot synthesis of 1,2,3-triazole derivatives by the ‘Click’reaction of alkyl halides or aryl boronic acids, sodium azide and terminal alkynes over a Cu/Al2O3 surface under ball-milling. Green Chem. 2013, 15, 389–397. [Google Scholar] [CrossRef]
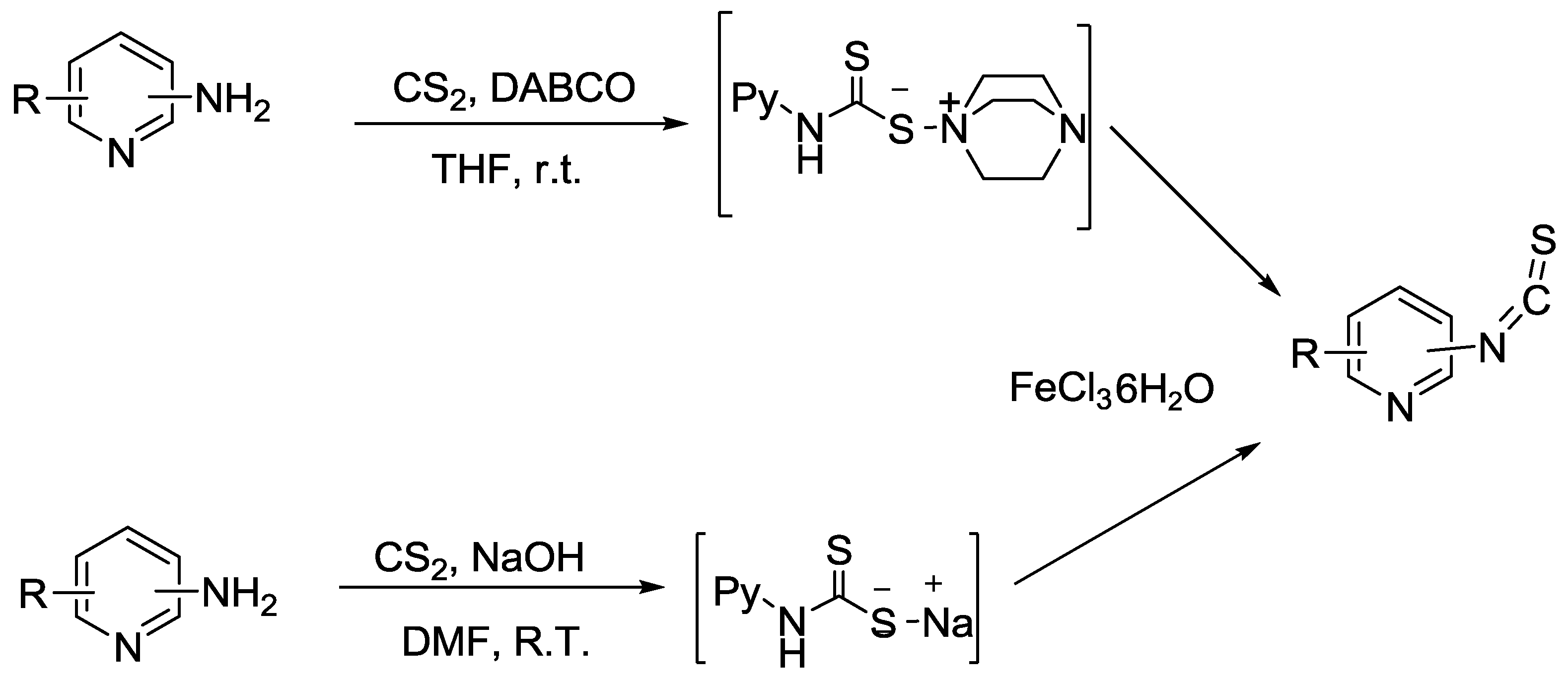
- Zhang, H.; Liu, R.-Q.; Liu, K.-C.; Li, Q.-B.; Li, Q.-Y.; Liu, S.-Z. A One-Pot Approach to Pyridyl Isothiocyanates from Amines. Molecules 2014, 19, 13631–13642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Li, Z.; Su, W. Synthesis of Quinolines by N-Deformylation and Aromatization via Solvent-Free, High.-Speed Ball Milling. Synth. Commun. 2013, 43, 361–374. [Google Scholar] [CrossRef]
- Maleki, A.; Javanshir, S.; Naimabadi, M. Facile synthesis of imidazo [1,2-a] pyridines via a one-pot three-component reaction under solvent-free mechanochemical ball-milling conditions. RSC Adv. 2014, 4, 30229–30232. [Google Scholar] [CrossRef]
- Wang, F.-J.; Xu, H.; Xin, M.; Zhang, Z. I2-mediated amination/cyclization of ketones with 2-aminopyridines under high-speed ball milling: Solvent- and metal-free synthesis of 2,3-substituted imidazo[1,2-a]pyridines and zolimidine. Mol. Divers. 2016, 20, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kausar, N.; Das, A.R. CuI–Zn(OAc)2 catalyzed C(sp2)–H activation for the synthesis of pyridocoumarins through an uncommon CuI–CuIII switching mechanism: A fast, solvent-free, combo-catalytic, ball milling approach. Tetrahedron Lett. 2017, 58, 2602–2607. [Google Scholar] [CrossRef]
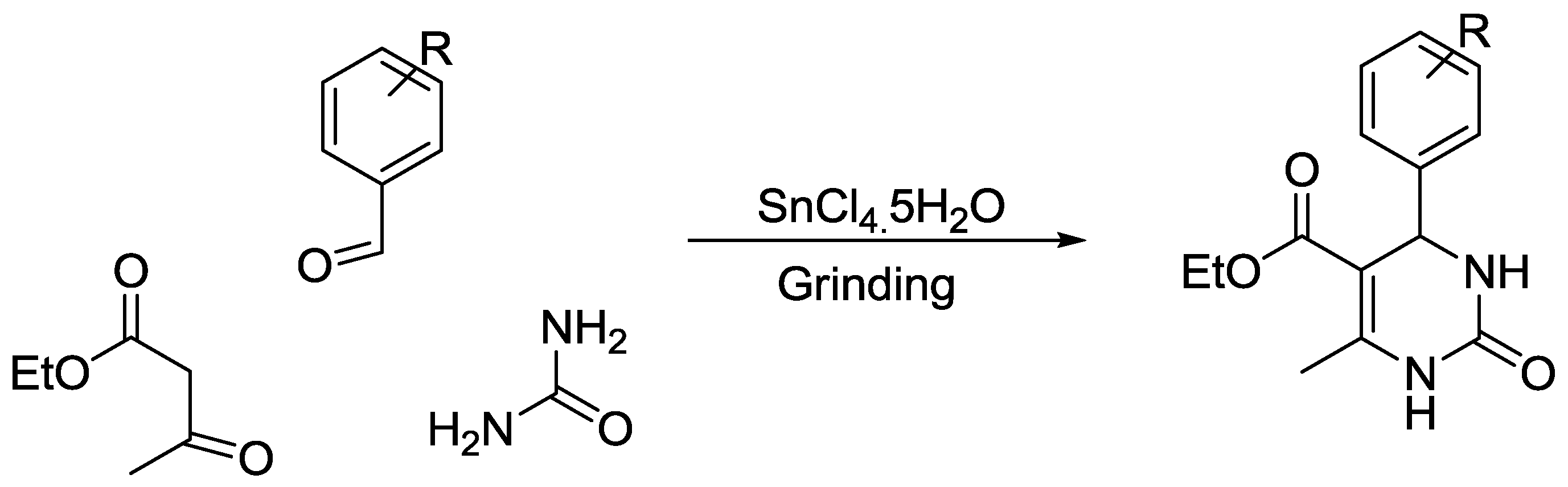
- Sahoo, P.K.; Bose, A.; Mal, P. Solvent-Free Ball-Milling Biginelli Reaction by Subcomponent Synthesis. Eur. J. Org. Chem. 2015, 2015, 6994–6998. [Google Scholar] [CrossRef]
- Sachdeva, H.; Saroj, R.; Khaturia, S.; Singh, H.L. Comparative studies of lewis acidity of alkyl-tin chlorides in multicomponent biginelli condensation using grindstone chemistry technique. J. Chil. Chem. Soc. 2012, 57, 1012–1016. [Google Scholar] [CrossRef]
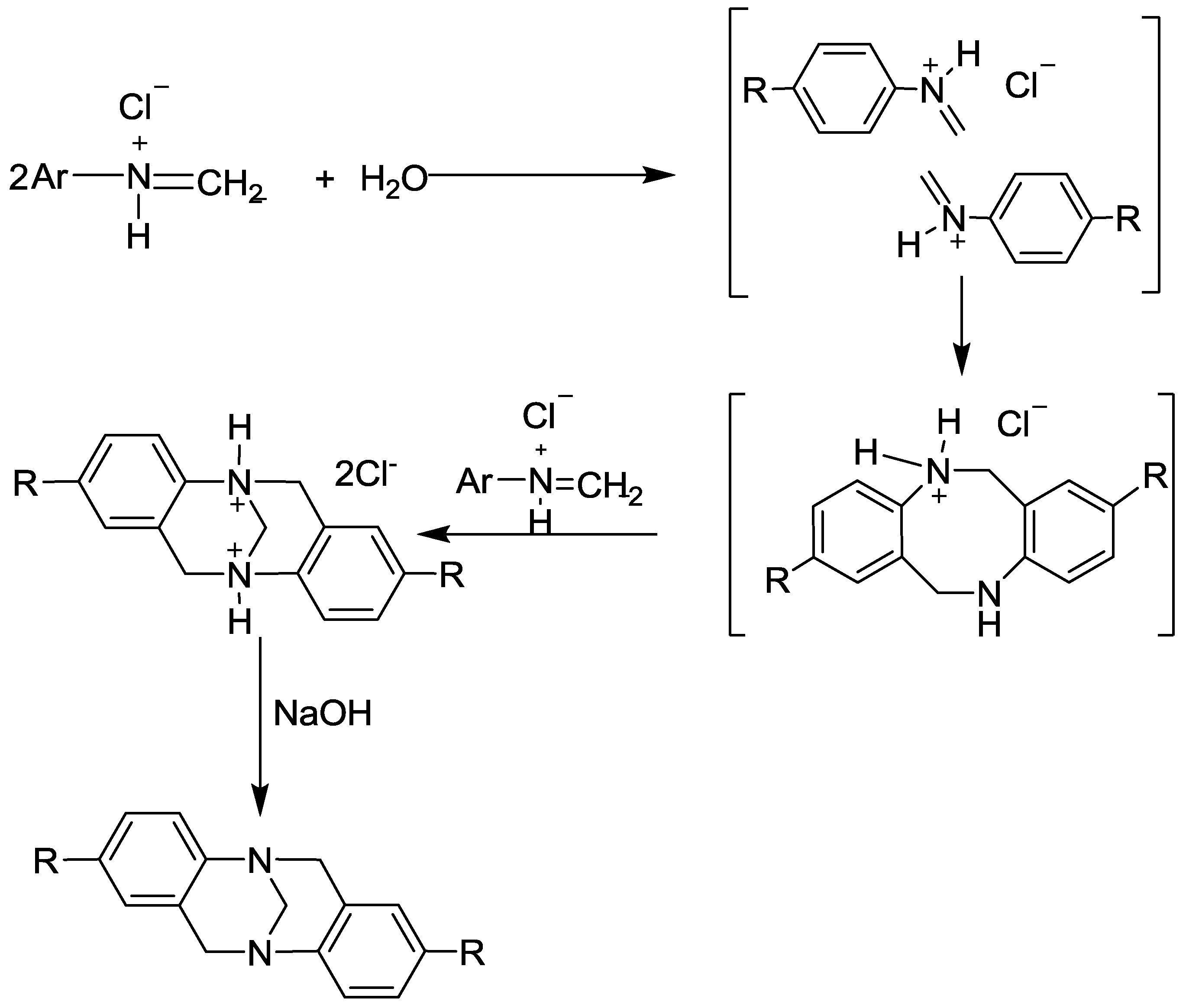
- Oliveira, P.F.; Haruta, N.; Chamayou, A.; Guidetti, B.; Baltas, M.; Tanaka, K.; Sato, T.; Baron, M. Comprehensive experimental investigation of mechanically induced 1, 4-diazines synthesis in solid state. Tetrahedron 2017, 73, 2305–2310. [Google Scholar] [CrossRef]
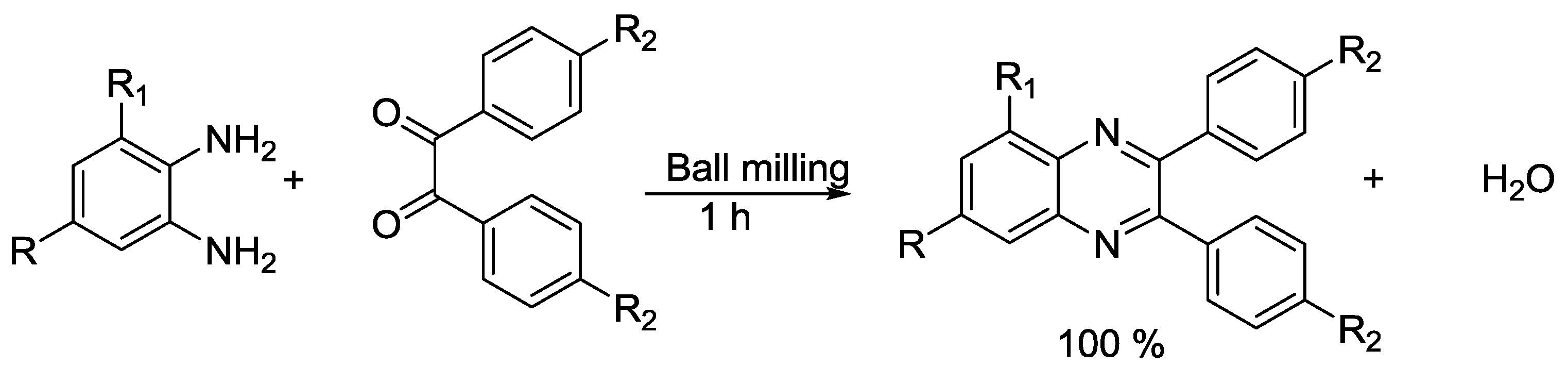
- Bhutia, Z.T.; Prasannakumar, G.; Das, A.; Biswas, M.; Chatterjee, A.; Banerjee, M. A Facile, Catalyst-Free Mechano-Synthesis of Quinoxalines and their In-Vitro Antibacterial Activity Study. ChemistrySelect 2017, 2, 1183–1187. [Google Scholar] [CrossRef]
- Kaupp, G.; Naimi-Jamal, M.R.; Schmeyers, J. Quantitative Reaction Cascades of Ninhydrin in the Solid State. Chemistry 2002, 8, 594–600. [Google Scholar] [CrossRef]
- Carlier, L.; Baron, M.; Chamayou, A.; Couarraze, G. Use of co-grinding as a solvent-free solid state method to synthesize dibenzophenazines. Tetrahedron Lett. 2011, 52, 4686–4689. [Google Scholar] [CrossRef]
- Etman, H.; Metwally, H.; Elkasaby, M.; Khalil, A.; Metwally, M. Green, two components highly efficient reaction of ninhydrin with aromatic amines, and malononitrile using ball-milling technique. Am. J. Org. Chem. 2011, 1, 10–13. [Google Scholar] [CrossRef]
- Sharifi, A.; Ansari, M.; Darabi, H.R.; Abaee, M.S. Synergistic promoting effect of ball milling and KF–alumina support for the green synthesis of benzothiazinones. Tetrahedron Lett. 2016, 57, 529–532. [Google Scholar] [CrossRef]
- Kaupp, G.; Schmeyers, J.; Boy, J. Iminium Salts in Solid-State Syntheses Giving 100% Yield. J. Prakt. Chem. 2000, 342, 269–280. [Google Scholar] [CrossRef]



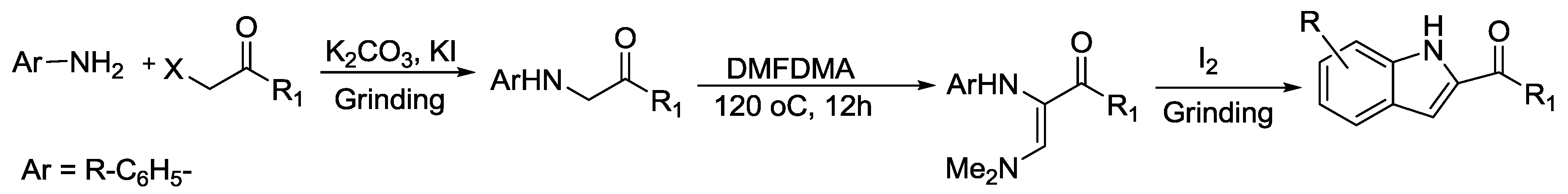


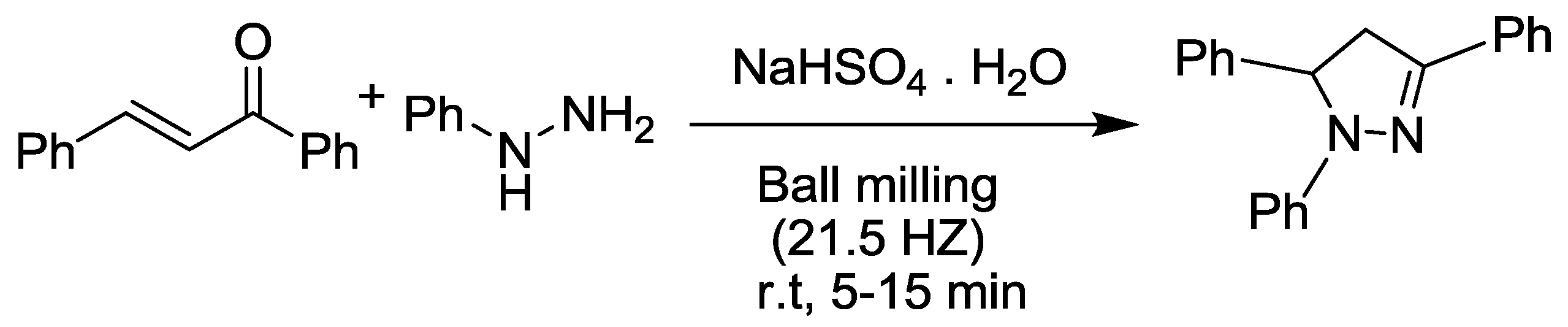





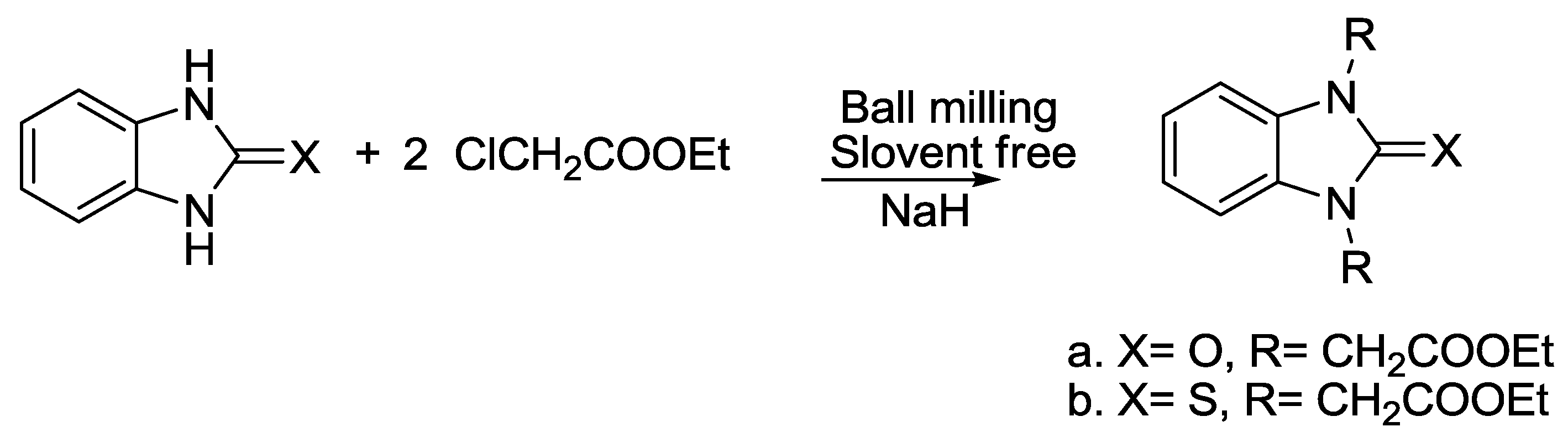




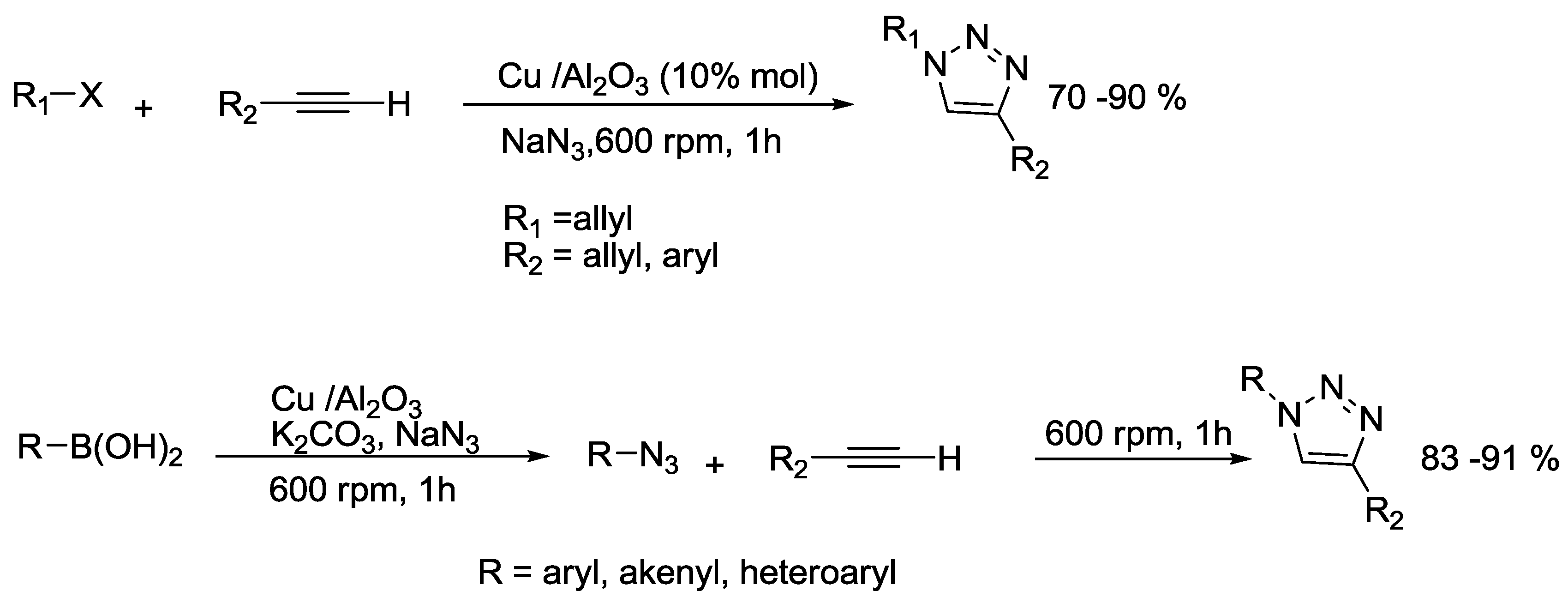
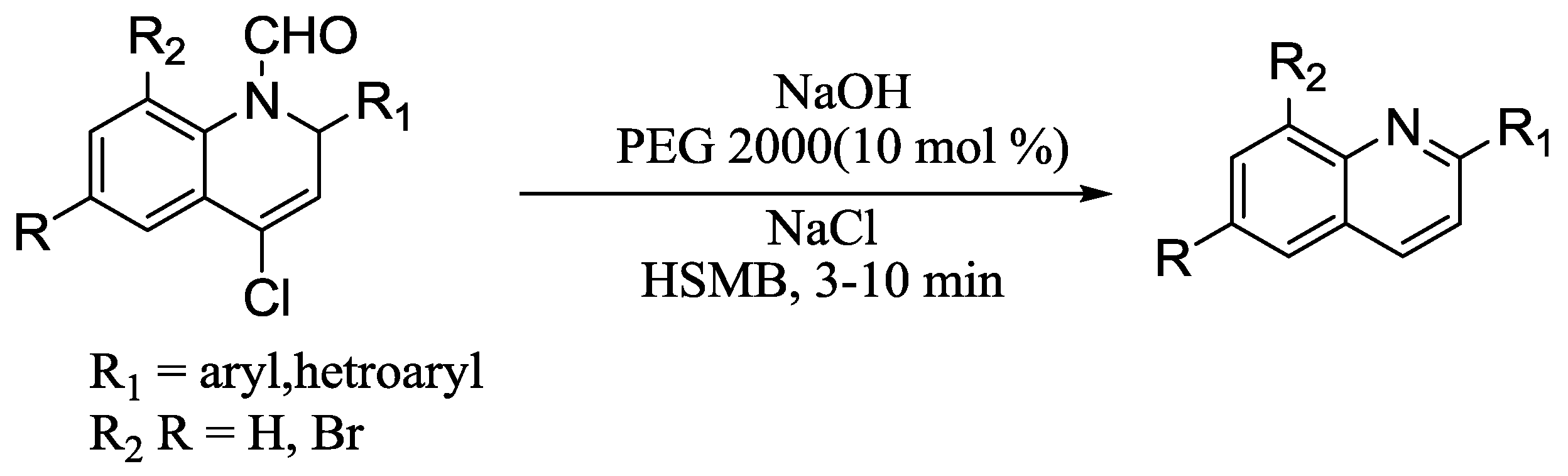









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, T.H.; Aboelnaga, A.; El-Atawy, M.A.; Hagar, M. Ball Milling Promoted N-Heterocycles Synthesis. Molecules 2018, 23, 1348. https://doi.org/10.3390/molecules23061348
El-Sayed TH, Aboelnaga A, El-Atawy MA, Hagar M. Ball Milling Promoted N-Heterocycles Synthesis. Molecules. 2018; 23(6):1348. https://doi.org/10.3390/molecules23061348
Chicago/Turabian StyleEl-Sayed, Taghreed H., Asmaa Aboelnaga, Mohamed A. El-Atawy, and Mohamed Hagar. 2018. "Ball Milling Promoted N-Heterocycles Synthesis" Molecules 23, no. 6: 1348. https://doi.org/10.3390/molecules23061348






