Ursolic Acid-Induced Apoptosis via Regulation of the PI3K/Akt and MAPK Signaling Pathways in Huh-7 Cells
Abstract
:1. Introduction
2. Results
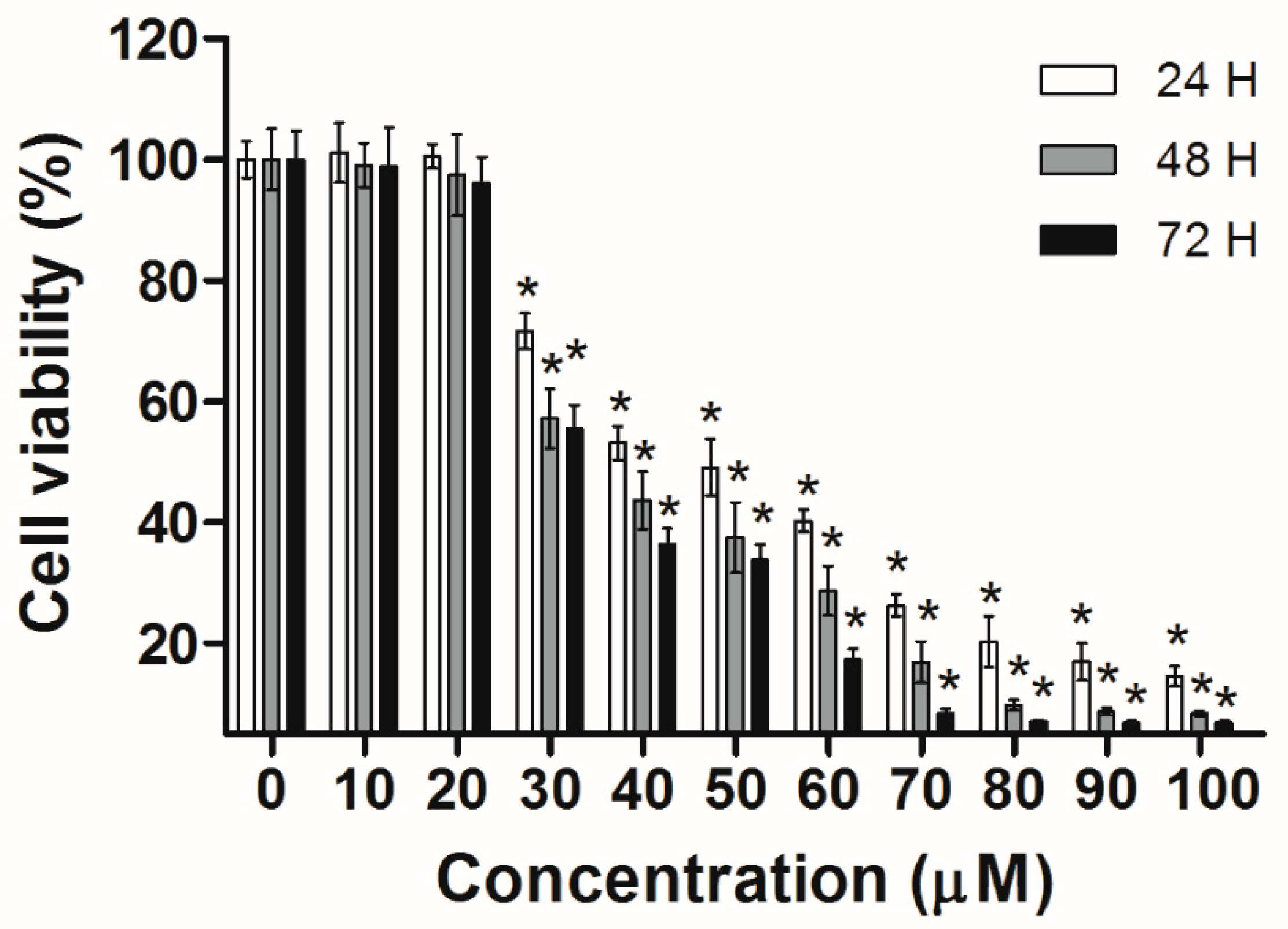
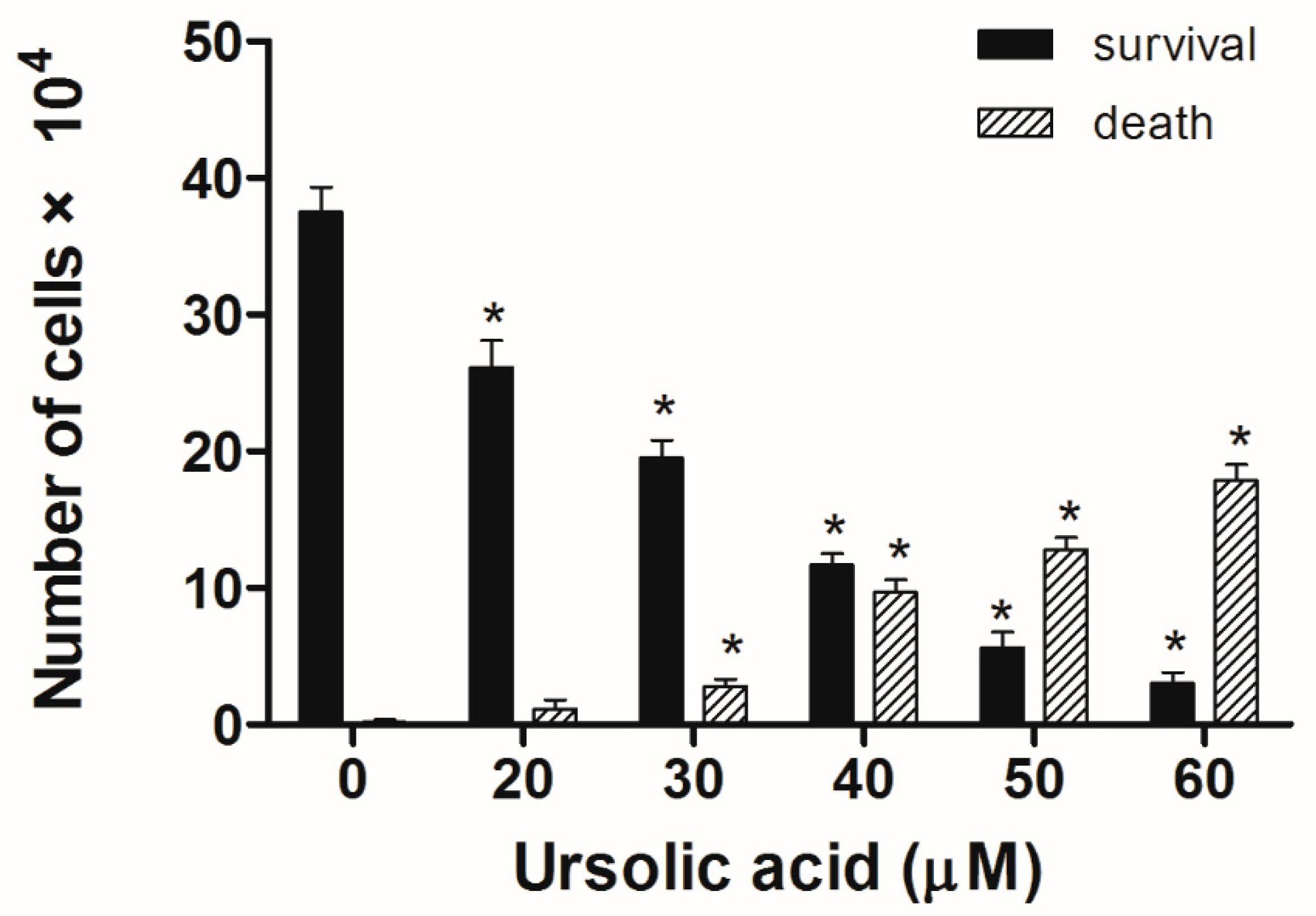
2.1. UA Inhibits the Growth of Huh-7 Cells
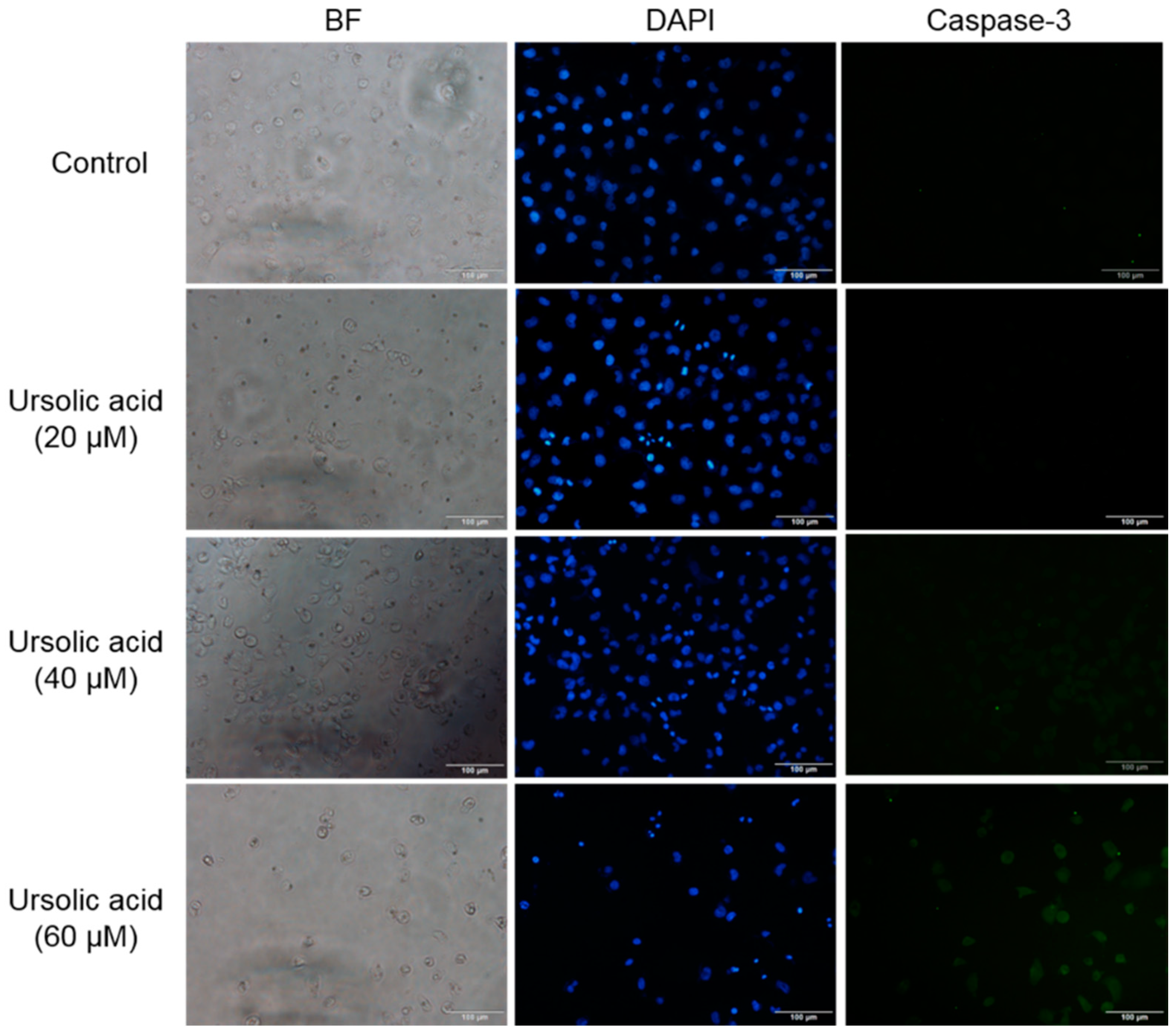
2.2. UA Increased the Expression of Caspase-3 in Huh-7 Cells
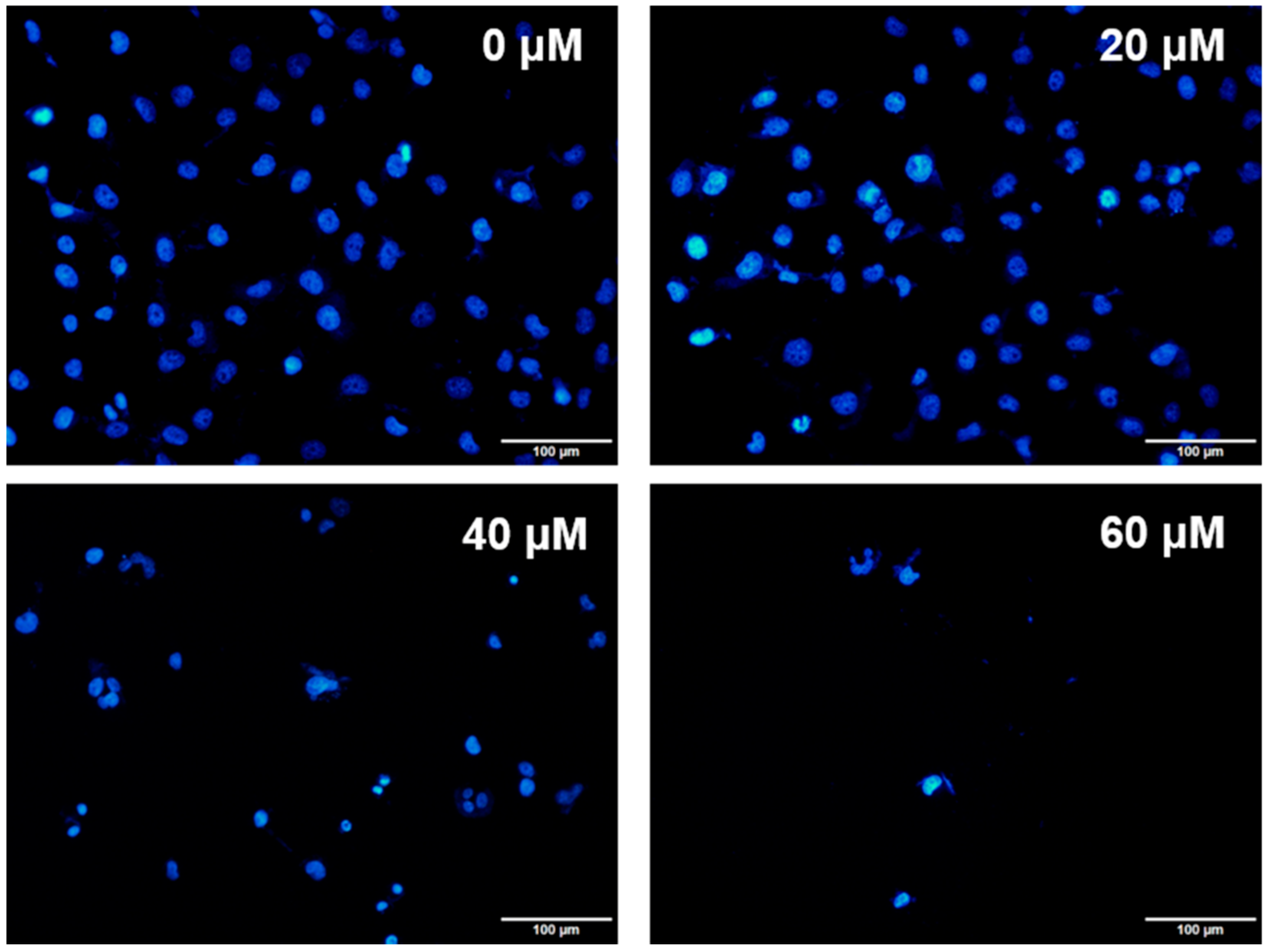
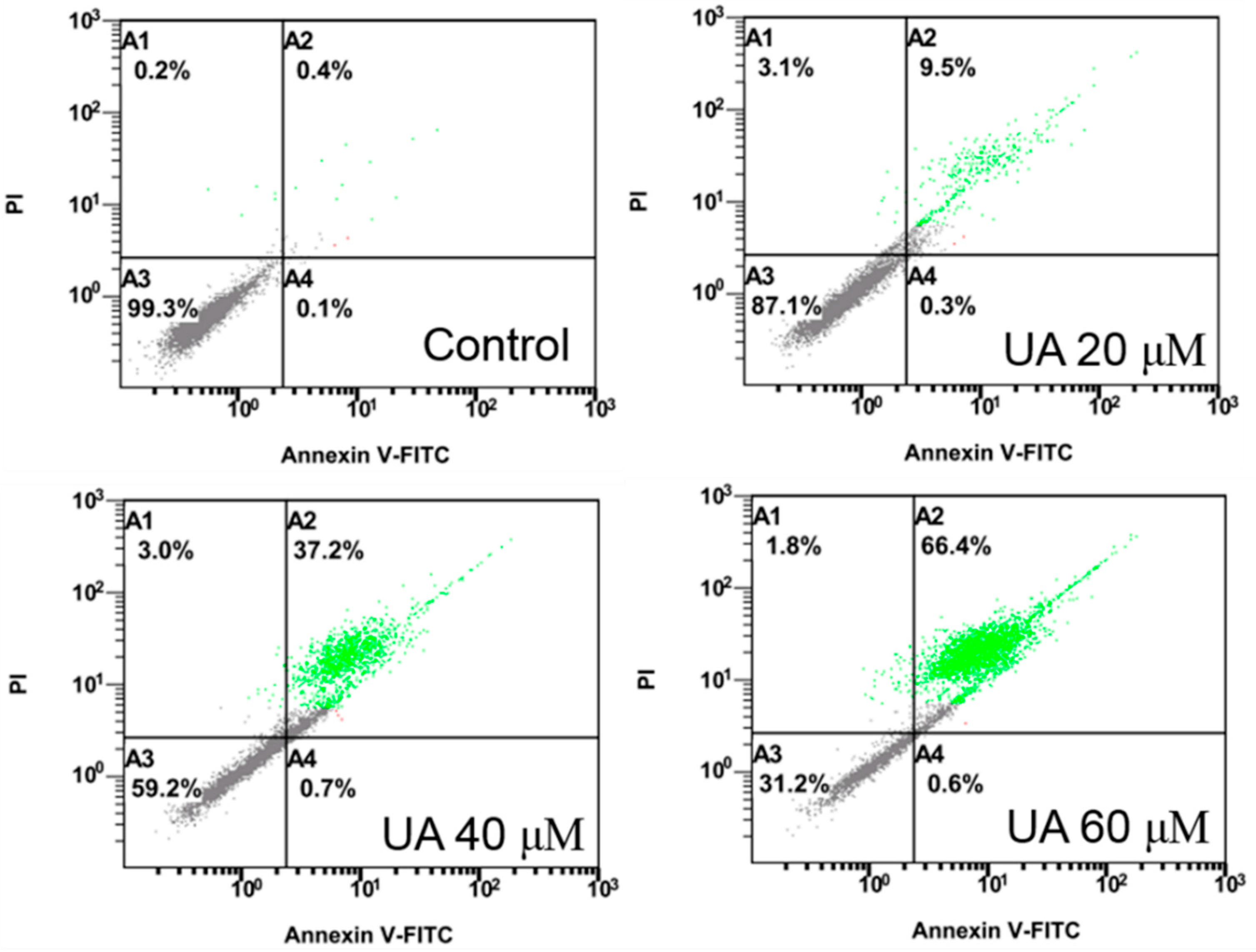
2.3. UA Induces Apoptosis in Huh-7 Cells
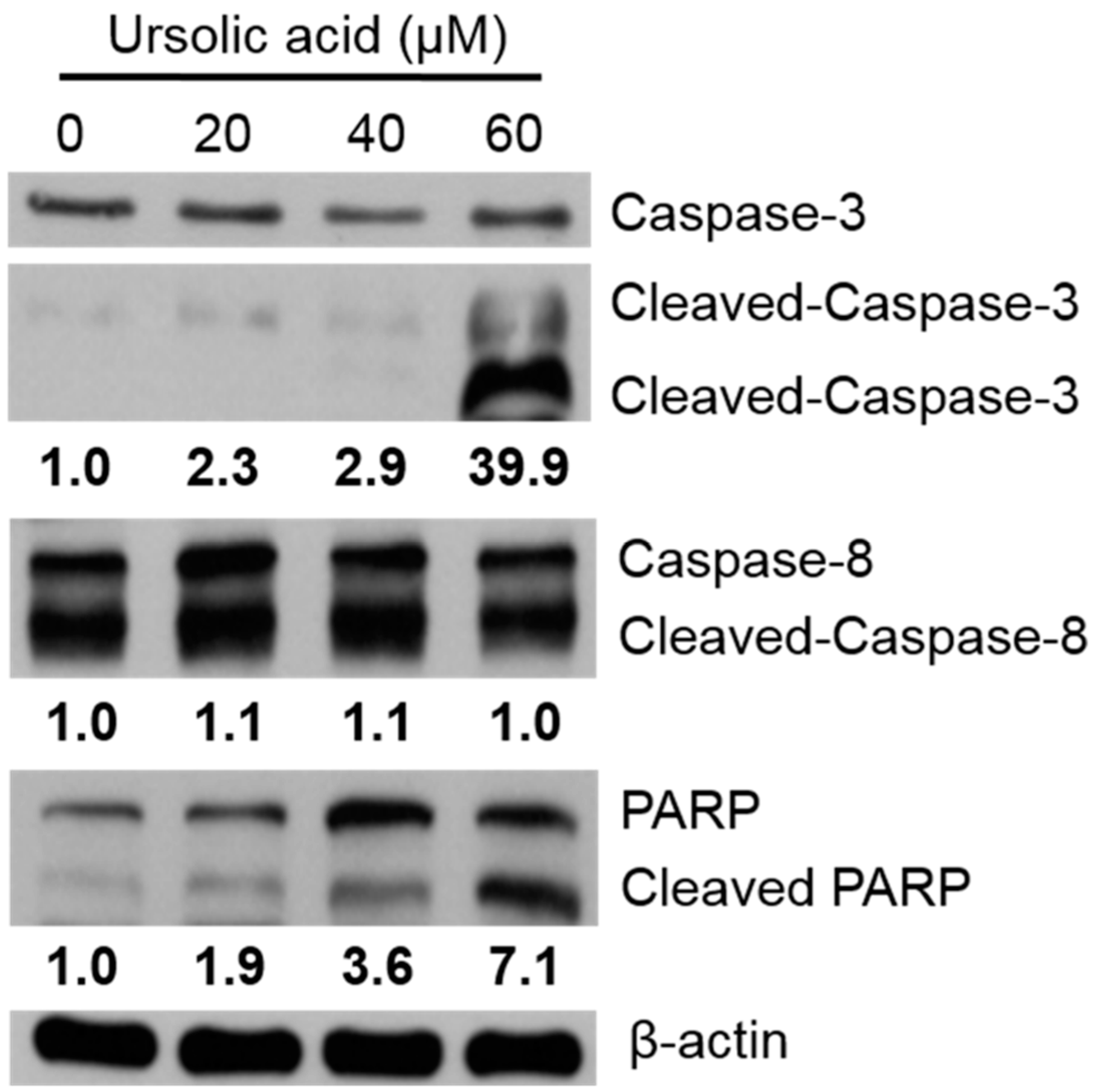
2.4. Caspases and PARP Expression Activated by UA
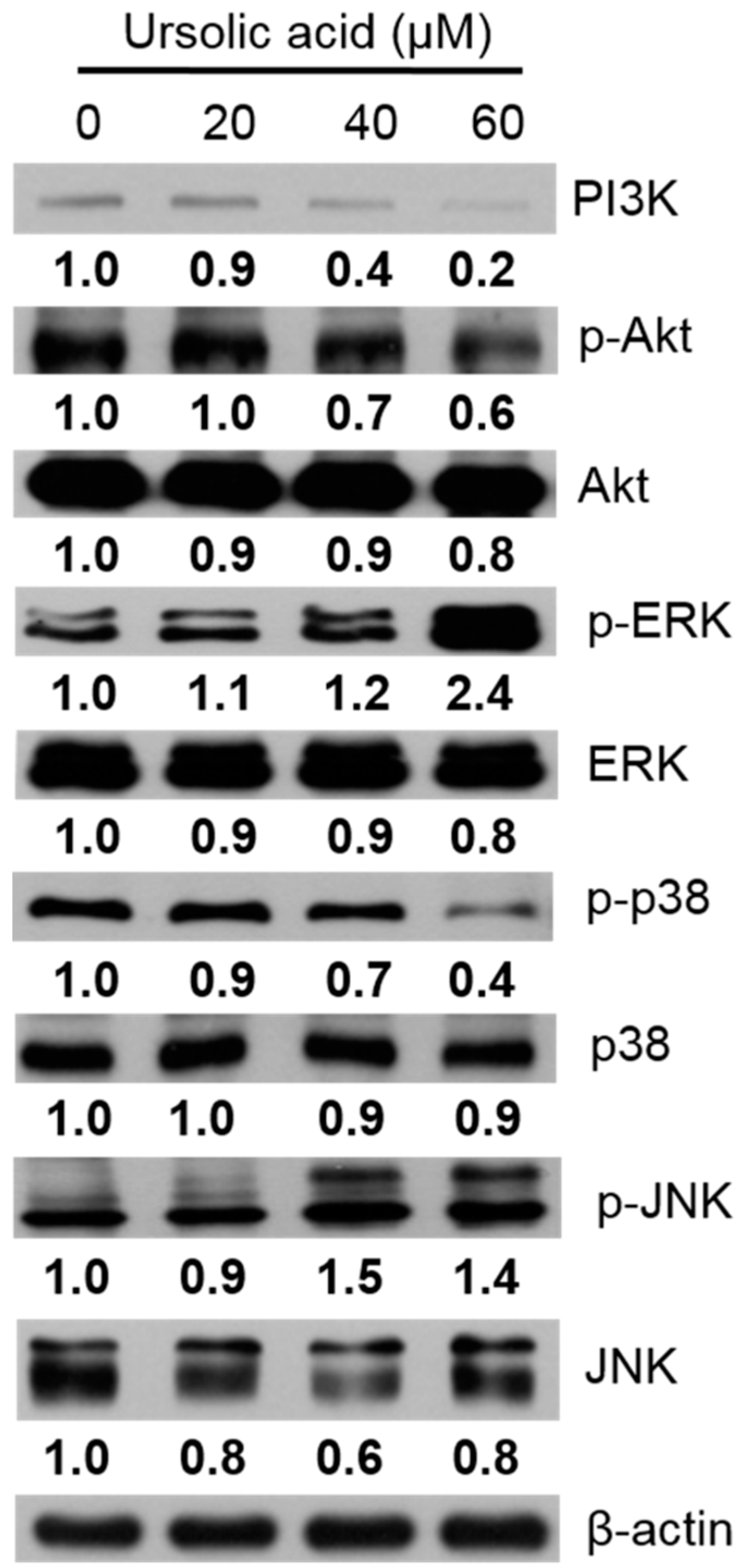
2.5. Apoptosis in Huh-7 Cells by Regulating the PI3K/Akt, p38 MAPK, ERK1/2, and JNK1/2 Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Line
4.3. Cell Cytotoxicity
4.4. Trypan Blue Assay
4.5. Immunocytochemical Staining
4.6. Annexin V/PI Double Staining Assay
4.7. Cell Lysis and Immunoblot Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EGF | epidermal growth factor |
| PI3K | phosphoinositide 3-kinase |
| MAPK | mitogen-activated protein kinase |
| UA | ursolic acid |
| p38 | p38 MAP kinase |
| ERK | extracellular signal-regulated kinase |
| JNK | cJun N-terminal kinase |
| PARP | poly (ADP-ribose) polymerase |
| TNF-α | tumor necrosis factor alpha |
| Bcl-2 | B-cell lymphoma 2 |
| TCTP | translationally controlled tumor protein |
References
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular carcinoma: Risk factors, diagnosis and treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dageforde, L.A.; Fowler, K.J.; Chapman, W.C. Liver transplantation for hepatocellular carcinoma: Current update on treatment and allocation. Curr. Opin. Organ Transplant. 2017, 22, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Kawabe, N.; Tomishige, H.; Nagata, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Recent advances in liver resection for hepatocellular carcinoma. Front. Surg. 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Baik, I.H.; Ye, S.K.; Lee, Y.H. Molecular targeted therapy for hepatocellular carcinoma: Present status and future directions. Biol. Pharm. Bull. 2015, 38, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Signaling pathway and molecular-targeted therapy for hepatocellular carcinoma. Dig. Dis. 2011, 29, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Huether, A.; Höpfner, M.; Sutter, A.P.; Schuppan, D.; Scherübl, H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J. Hepatol. 2005, 43, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, K.M.; Koprowski, S.; Kunnimalaiyaan, S.; Balamurugan, M.; Gamblin, T.C.; Kunnimalaiyaan, M. Potential molecular targeted therapeutics: Role of pi3-k/akt/mtor inhibition in cancer. Anticancer Agents Med. Chem. 2016, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-T.; Chi, C.-W. Emerging role of the peroxisome proliferator-activated receptor-gamma in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2014, 1, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Delire, B.; Starkel, P. The ras/mapk pathway and hepatocarcinoma: Pathogenesis and therapeutic implications. Eur. J. Clin. Investig. 2015, 45, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Pironi, A.M.; de Araujo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A review. Crit. Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (ua): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, A.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Preciado, L.M.; Rey-Suarez, P.; Henao, I.C.; Pereanez, J.A. Betulinic, oleanolic and ursolic acids inhibit the enzymatic and biological effects induced by a p-i snake venom metalloproteinase. Chem. Biol. Interact. 2018, 279, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pi, J.; Yang, F.; Wu, C.; Cheng, X.; Bai, H.; Huang, D.; Jiang, J.; Cai, J.; Chen, Z.W. Ursolic acid-loaded chitosan nanoparticles induce potent anti-angiogenesis in tumor. Appl. Microbiol. Biotechnol. 2016, 100, 6643–6652. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Ursolic acid-mediated changes in glycolytic pathway promote cytotoxic autophagy and apoptosis in phenotypically different breast cancer cells. Apoptosis 2017, 22, 800–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.X.; Li, Y.; Tian, D.D.; Liu, Y.; Nian, W.; Zou, X.; Chen, Q.Z.; Zhou, L.Y.; Deng, Z.L.; He, B.C. Ursolic acid inhibits proliferation and induces apoptosis by inactivating wnt/beta-catenin signaling in human osteosarcoma cells. Int. J. Oncol. 2016, 49, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; Lin, Y.; Liu, Y.; You, Y.; Yin, W. Ire1alpha-traf2-ask1 pathway is involved in cstmp-induced apoptosis and er stress in human non-small cell lung cancer a549 cells. Biomed. Pharmacother. 2016, 82, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Moon, A. Ursolic acid inhibits the invasive phenotype of snu-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Bernardazzi, C.; de Souza, H.S. Cell death and inflammatory bowel diseases: Apoptosis, necrosis, and autophagy in the intestinal epithelium. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, M.; Derry, W.B. Your neighbours matter—Non-autonomous control of apoptosis in development and disease. Cell Death Differ. 2016, 23, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in huh7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of xiap. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Koo, Y.; Ng, A.; Wei, Y.; Luby-Phelps, K.; Juraszek, A.; Xavier, R.J.; Cleaver, O.; Levine, B.; Amatruda, J.F. Autophagy is essential for cardiac morphogenesis during vertebrate development. Autophagy 2014, 10, 572–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of bcl2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Seo, J.; Kim, J.; Kim, J.H. Ursolic acid causes cell death in pc-12 cells by inducing apoptosis and impairing autophagy. Anticancer Res. 2018, 38, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Yang, L.; Mei, Y.; Long, H.; Zhang, X.; Zhang, J.; Qimuge, S.; Su, X. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, G.; Getzenberg, R.H.; Veltri, R.W. The upregulation of pi3k/akt and map kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J. Cell. Biochem. 2015, 116, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, D.; Rathore, K.; Cekanova, M. Phosphatidylinositol-3-kinase inhibitor induces chemosensitivity to a novel derivative of doxorubicin, ad198 chemotherapy in human bladder cancer cells in vitro. BMC Cancer 2015, 15, 927. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.L.; Wu, B.N.; Lin, Y.; Wang, J.; Fu, L.; Tang, Z.Y. Research progress of ursolic acid’s anti-tumor actions. Chin. J. Integr. Med. 2014, 20, 72–79. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound 3β-hydroxyurs-12-en-28-oic acid are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-C.; Chen, Y.-L.; Lin, P.-Y.; Chuang, W.-L. Ursolic Acid-Induced Apoptosis via Regulation of the PI3K/Akt and MAPK Signaling Pathways in Huh-7 Cells. Molecules 2018, 23, 2016. https://doi.org/10.3390/molecules23082016
Lee K-C, Chen Y-L, Lin P-Y, Chuang W-L. Ursolic Acid-Induced Apoptosis via Regulation of the PI3K/Akt and MAPK Signaling Pathways in Huh-7 Cells. Molecules. 2018; 23(8):2016. https://doi.org/10.3390/molecules23082016
Chicago/Turabian StyleLee, Kwong-Chiu, Yao-Li Chen, Ping-Yi Lin, and Wan-Ling Chuang. 2018. "Ursolic Acid-Induced Apoptosis via Regulation of the PI3K/Akt and MAPK Signaling Pathways in Huh-7 Cells" Molecules 23, no. 8: 2016. https://doi.org/10.3390/molecules23082016




