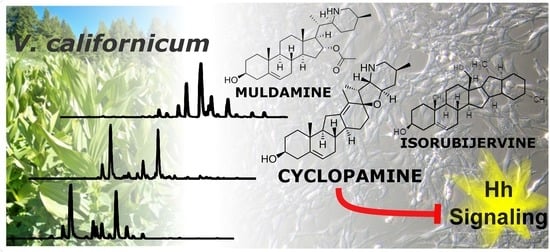
Native V. californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling
Abstract
1. Introduction
2. Results
2.1. Qualitative Comparison of V. californicum Alkaloids by Plant Part
2.2. Quantitative Analysis of V. californicum Alkaloids
2.3. Bioactivity Evaluation of Combined Standards and Plant Extracts
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
4.2. Sample Extraction and Preparation
4.3. Alkaloid Quantification
4.4. Alkaloid Identification
4.5. Cell Culture
4.6. Biological Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ingham, P.W. Hedgehog signaling in animal development: Paradigms and principles. Gene Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, T.; Carpenter, R.; Qasem, S.; Chan, M.; Lo, H.W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Finco, I.; Lapensee, C.R.; Krill, K.T.; Hammer, G.D. Hedgehog Signaling and Steroidogenesis. Annu. Rev. Physiol. 2015, 77, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Gene Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Pathi, S.; Pagan-Westphal, S.; Baker, D.P.; Garber, E.A.; Rayhorn, P.; Bumcrot, D.; Tabin, C.J.; Blake Pepinsky, R.; Williams, K.P. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 2001, 106, 107–117. [Google Scholar] [CrossRef]
- Chandler, C.M.; Mcdougal, O.M. Medicinal history of North American. Veratrum. Phytochem. Rev. 2013, 13, 671–694. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Katano, M. Hedgehog signaling pathway as a therapeutic target in various types of cancer. Can. Sci. 2011, 102, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Singh, R.R.; Vega, F. Aberrant Activation of the Hedgehog Signaling Pathway in Malignant Hematological Neoplasms. Am. J. Pathol. 2012, 180, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.E.; Furic, L.; Buchanan, G.; Larsson, O.; Pedersen, J.; Frydenberg, M.; Risbridger, G.P.; Taylor, R.A. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate 2013, 73, 1810–1823. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Onishi, H.; Nakano, K.; Nagamatsu, I.; Imaizumi, A.; Hattori, M.; Oda, Y.; Tanaka, M.; Katano, M. Hedgehog pathway as a therapeutic target for gallbladder cancer. Immunohistochemical staining for Gli1 in gallbladder cancer. Can. Sci. 2014, 105. [Google Scholar] [CrossRef]
- Onishi, H.; Kai, M.; Odate, S.; Iwasaki, H.; Morifuji, Y.; Ogino, T.; Morisaki, T.; Nakashima, Y.; Katano, M. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of Smo transcription in pancreatic cancer. Can. Sci. 2011, 102, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.M.; Strange, R.C.; Lear, J.T. Basal cell carcinoma. BMJ 2003, 327, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Oro, A.E.; Higgins, K.M.; Hu, Z.; Bonifas, J.M.; Epstein, E.H.; Scott, M.P. Basal Cell Carcinomas in Mice Overexpressing Sonic Hedgehog. Science 1997, 276, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Murone, M.; Luoh, S.M.; Ryan, A.; Gu, Q.; Zhang, C.; Bonifas, J.M.; Lam, C.W.; Hynes, M.; Goddard, A.; et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 1998, 391, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.J.; Pau, G.; Dijkgraaf, G.J.; Basset-Seguin, N.; Modrusan, Z.; Januario, T.; Tsui, V.; Durham, A.M.; Dlugosz, A.A.; Haverty, P.M.; et al. Genomic Analysis of Smoothened Inhibitor Resistance in Basal Cell Carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.G.; O’Kane, G.M.; Kelly, C.M. Efficacy and safety of vismodegib: A new therapeutic agent in the treatment of basal cell carcinoma. Expert Opin. Drug Saf. 2014, 13, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Oro, A.E. Initial Assessment of Tumor Regrowth after Vismodegib in Advanced Basal Cell Carcinoma. Arch. Dermatol. 2012, 148, 1324–1325. [Google Scholar] [CrossRef] [PubMed]
- Pricl, S.; Cortelazzi, B.; Col, V.D.; Marson, D.; Laurini, E.; Fermeglia, M.; Licitra, L.; Pilotti, S.; Bossi, P.; Perrone, F. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol. Oncol. 2014, 9, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yauch, R.L.; Dijkgraaf, G.J.P.; Alicke, B.; Januario, T.; Ahn, C.P.; Holcomb, T.; Pujara, K.; Stinson, J.; Callahan, C.A.; Tang, T.; et al. Smoothened Mutation Confers Resistance to a Hedgehog Pathway Inhibitor in Medulloblastoma. Science 2009, 326, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, F.; Li, X.; Xu, S.; Huang, W.; Ye, Y. Three new alkaloids from Veratrum grandiflorum Loes with inhibition activities on Hedgehog pathway. Bioorg. Med. Chem. Lett. 2016, 26, 4735–4738. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Sayed, K.A.E. The Veratrum alkaloids jervine, veratramine, and their analogues as prostate cancer migration and proliferation inhibitors: Biological evaluation and pharmacophore modeling. Med. Chem. Res. 2013, 22, 4775–4786. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.Q.; Zhang, X. Cyclopamine, a Naturally Occurring Alkaloid, and Its Analogues May Find Wide Applications in Cancer Therapy. Curr. Top. Med. Chem. 2013, 13, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Strand, M.F.; Krapp, A.; Rise, F.; Petersen, D.; Krauss, S. Hedgehog antagonist cyclopamine isomerizes to less potent forms when acidified. J. Pharm. Biomed. Anal. 2010, 52, 707–713. [Google Scholar] [CrossRef] [PubMed]
- McNeal, D.W.; Shaw, S.D. Veratrum. In Flora of North America North of Mexico; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2002; Volume 26, pp. 72–76. [Google Scholar]
- Cooper, M.K.; Porter, J.A.; Young, K.E.; Beachy, P.A. Teratogen-Mediated Inhibition of Target Tissue Response to Shh Signaling. Science 1998, 280, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K. I only have eye for ewe: The discovery of cyclopamine and development of Hedgehog pathway-targeting drugs. Nat. Prod. Rep. 2016, 33, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Heretsch, P.; Tzagkaroulaki, L.; Giannis, A. Cyclopamine and Hedgehog signaling: Chemistry, Biology, Medical Perspectives. Angew. Chem. Int. Ed. 2010, 49, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.M.; Habig, J.W.; Fisher, A.A.; Ambrose, K.V.; Jimenez, S.T.; McDougal, O.M. Improved extraction and complete mass spectral characterization of steroidal alkaloids from Veratrum californicum. Nat. Prod. Commun. 2013, 8, 1059–1064. [Google Scholar] [PubMed]
- Turner, M.W.; Cruz, R.; Mattos, J.; Baughman, N.; Elwell, J.; Fothergill, J.; Nielsen, A.; Brookhouse, J.; Bartlett, A.; Malek, P.; et al. Cyclopamine bioactivity by extraction method from Veratrum californicum. Bioorg. Med. Chem. 2016, 24, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, H. Steroidal alkaloids from roots of Solanum spirale. Phytochemistry 1996, 43, 705–707. [Google Scholar] [CrossRef]
- Wilson, S.R.; Strand, M.F.; Krapp, A.; Rise, F.; Herstad, G.; Malterud, K.E.; Krauss, S. Hedgehog antagonists cyclopamine and dihydroveratramine can be mistaken for each other in Veratrum album. J. Pharm. Biomed. Anal. 2010, 53, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Keeler, R.F. Teratogenic compounds of Veratrum californicum (Durand) VII. The structure of the glycosidic alkaloid cycloposine. Steroids 1969, 13, 579–588. [Google Scholar] [CrossRef]
- Keeler, R.; Binns, W. Teratogenic compounds of Veratrum californicum (Durand) III. Malformations of the veratramine-induced type from ingestion of plant or roots. Proc. Soc. Exp. Biol. Med. 1967, 126, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Keeler, R.F. Teratogenic effects of cyclopamine and jervine in rats, mice and hamsters. Proc. Soc. Exp. Biol. 1975, 149, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Chen, J.K.; Cooper, M.K.; Wang, B.; Mann, R.K.; Milenkovic, L.; Scott, M.P.; Beachy, P.A. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 2000, 406, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Peak | Retention Time (min) | m/z | Molecular Formula | Alkaloid |
|---|---|---|---|---|
| 1 | 12.8 | 576.3836 | C33H53NO7 | N/A |
| 2 | 13.9 | 572.3530 | C33H49NO7 | Veratrosine |
| 3 | 14.6 | 574.3699 | C33H51NO7 | Cycloposine |
| 4 | 14.9 | 414.3337 | C27H43NO2 | N/A 1 |
| 5 | 15.7 | 430.3282 | C27H43NO3 | N/A |
| 6 | 16.6 | 428.3136 | C27H41NO3 | N/A |
| 7 | 16.7 | 576.3846 | C33H53NO7 | N/A |
| 8 | 16.9 | 410.3021 | C27H39NO2 | Veratramine |
| 9 | 17.4 | 410.3023 | C27H39NO2 | N/A 2 |
| 10 | 18.7 | 412.3186 | C27H41NO2 | Cyclopamine |
| 11 | 19.5 | 412.3184 | C27H41NO2 | N/A 3 |
| 12 | 19.7 | 414.3342 | C27H43NO2 | Isorubijervine |
| 13 | 21.1 | 458.3587 | C29H47NO3 | Muldamine |
| 14 | 23.4 | 400.3550 | C27H45NO | N/A |
| 15 | 24.5 | 456.3446 | C29H45NO3 | N/A |
| Plant Part | Cyclopamine | Veratramine | Muldamine | Isorubijervine |
|---|---|---|---|---|
| Leaf | 0.21 ± 0.02 | 0.09 ± 0.01 | Not Detected | Not Detected |
| Stem | 3.23 ± 0.16 | 1.33 ± 0.13 | 0.36 ± 0.06 | 1.00 ± 0.08 |
| Root/Rhizome | 7.38 ± 0.08 | 3.07 ± 0.14 | 3.47 ± 0.23 | 2.92 ± 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, M.W.; Cruz, R.; Elwell, J.; French, J.; Mattos, J.; McDougal, O.M. Native V. californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling. Molecules 2018, 23, 2222. https://doi.org/10.3390/molecules23092222
Turner MW, Cruz R, Elwell J, French J, Mattos J, McDougal OM. Native V. californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling. Molecules. 2018; 23(9):2222. https://doi.org/10.3390/molecules23092222
Chicago/Turabian StyleTurner, Matthew W., Roberto Cruz, Jordan Elwell, John French, Jared Mattos, and Owen M. McDougal. 2018. "Native V. californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling" Molecules 23, no. 9: 2222. https://doi.org/10.3390/molecules23092222
APA StyleTurner, M. W., Cruz, R., Elwell, J., French, J., Mattos, J., & McDougal, O. M. (2018). Native V. californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling. Molecules, 23(9), 2222. https://doi.org/10.3390/molecules23092222