Folic Acid Has a Protective Effect on Retinal Vascular Endothelial Cells against High Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compound
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Cell Viability Assay
2.5. Migration Assay
2.6. Tube Formation Assay
2.7. BrdU Assay
2.8. Molecular Docking
2.9. Western Blot Analysis
2.10. Real-Time PCR Assay
2.11. Statistical Analysis
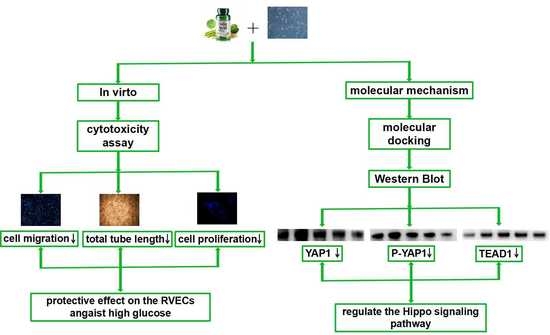
3. Results
3.1. The Cytotoxicity of Folic Acid
3.2. The Effects of Folic Acid on the Viability of High Glucose (HG)-Treated RVECs
3.3. The Effects of Folic Acid on the Migration of HG-Treated RVECs
3.4. The Effects of Folic Acid on the Tube Formation of HG-Treated RVECs
3.5. The Effects of Folic Acid on the Proliferation of HG-Treated RVECs
3.6. The Interaction of Folic Aicd with TEAD1, YAP1, DLL1 and Notch2
3.7. The Effects of Folic Acid on the mRNA Expression of TEAD1 and DLL1
3.8. The Effects of Folic Acid on the Protein Expression of TEAD1, YAP1, P-YAP1, DLL1 and Notch2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
Abbreviations
| DR | Diabetic retinopathy |
| PDR | Proliferative diabetic retinopathy |
| NPDR | Nonproliferative diabetic retinopathy |
| DWR | Diabetic without retinopathy |
| RVEC | Retinal vascular endothelial cell |
| OD | Optical density |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| RT | Room temperature |
| PDB | Protein Data Bank |
| SDS | Sodium dodecyl sulfate |
| DDT | Dichlorodiphenyltrichloroethane |
| cDNA | Complementary DNA |
| ANOVA | One-way analysis of variance |
| LSD | Least significant difference |
| VSMC | Vascular smooth muscle cell |
| PDGF-BB | Platelet derived growth factor |
| HUVEC | Human umbilical venous endothelial cell |
| NSC | Neural stem cell |
| CRC | Colorectal cancer |
| NG | Normal glucose |
| HG | High glucose |
References
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Saaddine, J.B.; Honeycutt, A.A.; Narayan, K.M.; Zhang, X.; Klein, R.; Boyle, J.P. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch. Ophthalmol. 2008, 126, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Ren, A. Folic acid prevents neural tube defects. J. Peking Univ. 2014, 46, 343–346. [Google Scholar]
- Chilom, C.G.; Bacalum, M.; Stanescu, M.M.; Florescu, M. Insight into the interaction of human serum albumin with folic acid: A biophysical study. Spectrochim. Acta A 2018, 204, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Gagliano, C.; Salomone, S.; Giordano, M.; Bucolo, C.; Pappalardo, A.; Drago, F.; Caraci, F.; Avitabile, T.; Motta, M. Folate status in type 2 diabetic patients with and without retinopathy. Clin. Ophthalmol. 2015, 9, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.; Sechi, E.; Fois, C.; Kumar, N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr. Rev. 2016, 74, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zeng, G.; Zhang, Y.; Li, Q.; Zhang, J.; Bai, Z.; Yang, K. Association between homocysteine level and the risk of diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Smolek, M.K.; Notaroberto, N.F.; Jaramillo, A.G.; Pradillo, L.R. Intervention with vitamins in patients with nonproliferative diabetic retinopathy: A pilot study. Clin. Ophthalmol. 2013, 7, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.L.; Powers, R. Application of NMR and molecular docking in structure-based drug discovery. Top. Curr. Chem. 2012, 326, 1–34. [Google Scholar] [PubMed]
- Chen, M.; Yang, F.; Yang, X.; Lai, X.; Gao, Y. Systematic Understanding of Mechanisms of a Chinese Herbal Formula in Treatment of Metabolic Syndrome by an Integrated Pharmacology Approach. Int. J. Mol. Sci. 2016, 17, 2114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Yao, X.; Li, D.; Xu, L.; Li, Y.; Tian, S.; Hou, T. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.N. Virtual Screening and Molecular Dynamics Simulation of α-Glucosidase Inhibitors. Masteral Dissertation, Dalian University of Technology, Dalian, China, 2016. [Google Scholar]
- Shen, H.; Rong, H. Pterostilbene impact on retinal endothelial cells under high glucose environment. Int. J. Clin. Exp. Pathol. 2015, 8, 12589–12594. [Google Scholar] [PubMed]
- Wu, Y.; Zhang, Q.; Zhang, R. Kaempferol targets estrogen-related receptor alpha and suppresses the angiogenesis of human retinal endothelial cells under high glucose conditions. Exp. Ther. Med. 2017, 14, 5576–5582. [Google Scholar] [PubMed]
- Pan, S.; Lin, H.; Luo, H.; Gao, F.; Meng, L.; Zhou, C.; Jiang, C.; Guo, Y.; Ji, Z.; Chi, J.; et al. Folic acid inhibits dedifferentiation of PDGF-BB-induced vascular smooth muscle cells by suppressing mTOR/P70S6K signaling. Am. J. Transl. Res. 2017, 9, 1307–1316. [Google Scholar] [PubMed]
- Lin, S.Y.; Lee, W.R.; Su, Y.F.; Hsu, S.P.; Lin, H.C.; Ho, P.Y.; Hou, T.C.; Chou, Y.P.; Kuo, C.T.; Lee, W.S. Folic acid inhibits endothelial cell proliferation through activating the cSrc/ERK 2/NF-ĸB/p53 pathway mediated by folic acid receptor. Angiogenesis 2012, 15, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.C.; Lin, J.J.; Wen, H.C.; Chen, L.C.; Hsu, S.P.; Lee, W.S. Folic acid inhibits endothelial cell migration through inhibiting the RhoA activity mediated by activating the folic acid receptor/cSrc/p190RhoGAP-signaling pathway. Biochem. Pharmacol. 2013, 85, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.F.; Brockton, N.T.; Bakkar, A.; Liu, S.; Wen, J.; Weljie, A.M.; Bismar, T.A. Elevated physiological levels of folic acid can increase in vitro growth and invasiveness of prostate cancer cells. BJU Int. 2012, 109, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, M.; Luo, S.; Liu, H.; Gao, Y.; Wilson, J.X.; Huang, G. DNA methyltransferase mediates dose-dependent stimulation of neural stem cell proliferation by folate. J. Nutr. Biochem. 2013, 24, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Miranda, D.; Bunout, D.; Ronco, A.M.; de la Maza, M.P.; Hirsch, S. Folates Induce Colorectal Carcinoma HT29 Cell Line Proliferation Through Notch1 Signaling. Nutr. Cancer 2015, 67, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, Z. Downregulation of YAP inhibits proliferation and induces apoptosis in Eca-109 cells. Exp. Ther. Med. 2018, 15, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Shi, H.; Zhu, R.; Li, L.; Qin, B.; Kang, L.; Chen, H.; Guan, H. Inhibition of YAP ameliorates choroidal neovascularization via inhibiting endothelial cell proliferation. Mol. Vis. 2018, 24, 83–93. [Google Scholar] [PubMed]
- Li, X.; Liu, Y.; Zhang, C.; Niu, Q.; Wang, H.; Che, C.; Xie, M.; Zhou, B.; Xu, Y.; Zhang, Q.; et al. Stiehopus japonieus acidic mucopolysaccharide inhibits the proliferation of pancreatic cancer SW1990 cells through Hippo-YAP pathway. Oncotarget 2017, 8, 16356–16366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.; Lv, X.; Hua, G.; He, C.; Dong, J.; Lele, S.M.; Li, D.W.; Zhai, Q.; Davis, J.S.; Wang, C. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr. Relat. Cancer 2014, 21, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Lin, F.; Wu, W.; Huang, W.; Cai, Z. Transcriptional cofactor Mask2 is required for YAP-induced cell growth and migration in bladder cancer cell. J. Cancer 2016, 7, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Hindley, C.J.; Condurat, A.L.; Menon, V.; Thomas, R.; Azmitia, L.M.; Davis, J.A.; Pruszak, J. The Hippo pathway member YAP enhances human neural crest cell fate and migration. Sci. Rep. 2016, 6, 23208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig. 2017, 127, 3441–3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulev, Y.; Fauny, J.D.; Gonzalez-Marti, B.; Flagiello, D.; Silber, J.; Zider, A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 2008, 18, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Zheng, Y.; Dong, J.; Pan, D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 2008, 14, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, F.; Zhang, Q.; Chen, Y.; Wang, B.; Jiang, J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 2008, 14, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilev, A.; Kaneko, K.J.; Shu, H.; Zhao, Y.; DePamphilis, M.L. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001, 15, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, F.; Wang, Y.; Li, T.; Xiu, P.; Zhong, J.; Sun, X.; Li, J. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017, 108, 478–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Gou, J.; Jia, J.; Yi, T.; Cui, T.; Li, Z. Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. Onco. Targets Ther. 2016, 9, 5371–5381. [Google Scholar] [PubMed]
- Yu, M.; Zhang, W. TEAD1 enhances proliferation via activating SP1 in colorectal cancer. Biomed. Pharmacother. 2016, 83, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Kiyonari, H.; Ukita, K.; Nishioka, N.; Imuta, Y.; Sasaki, H. Redundant Roles of Tead1 and Tead2 in Notochord Development and the Regulation of Cell Proliferation and Survival. Mol. Cell Biol. 2008, 28, 3177–3189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |






| Sequences | Sequences, 5’–3’ |
|---|---|
| TEAD1 | Forward: ACCTCTTGGCAGTACAGTATTC |
| Reverse: CACTTTAAAGCCAACACTTAGAACA | |
| YAP1 | Forward: TGAACAAACGTCCAGCAAGATAC |
| Reverse: CAGCCCCCAAAATGAACAGTAC | |
| DLL1 | Forward: TGAACGACTTCTCCTGCACC |
| Reverse: GATGCTTCTCCACTGCTGACG | |
| Notch2 | Forward: CTGGTGCCTATTGTGACGTG |
| Reverse: TTCAACAAGCACACCTCTCCT | |
| β-actin | Forward: AGCCATGTACGTAGCCATCC |
| Reverse: TCTCAGCTGTGGTGGTGAAG |
| Step | Temperature, °C | Duration |
|---|---|---|
| 1 | 95 | 10 min |
| 2 | 95 | 15 sec |
| 3 | 60 | 60 sec |
| Steps 2–3 | - | 40 cycles |
| Component | Signaling Pathway | Target Protein | Docking Scores |
|---|---|---|---|
| folic acid | Hippo | TEAD1 | −8.84 |
| YAP1 | −6.08 | ||
| Notch | DLL1 | −6.25 | |
| Notch2 | −5.77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xing, W.; Song, Y.; Li, H.; Liu, Y.; Wang, Y.; Li, C.; Wang, Y.; Wu, Y.; Han, J. Folic Acid Has a Protective Effect on Retinal Vascular Endothelial Cells against High Glucose. Molecules 2018, 23, 2326. https://doi.org/10.3390/molecules23092326
Wang Z, Xing W, Song Y, Li H, Liu Y, Wang Y, Li C, Wang Y, Wu Y, Han J. Folic Acid Has a Protective Effect on Retinal Vascular Endothelial Cells against High Glucose. Molecules. 2018; 23(9):2326. https://doi.org/10.3390/molecules23092326
Chicago/Turabian StyleWang, Zhenglin, Wei Xing, Yongli Song, Hongli Li, Yonggang Liu, Yong Wang, Chun Li, Yun Wang, Yan Wu, and Jing Han. 2018. "Folic Acid Has a Protective Effect on Retinal Vascular Endothelial Cells against High Glucose" Molecules 23, no. 9: 2326. https://doi.org/10.3390/molecules23092326