LC–Q–TOF–MS/MS Identification of Specific Non-Meat Proteins and Peptides in Beef Burgers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Specific Non-Meat Proteins
2.2. Identification of Specific Peptide Markers
3. Materials and Methods
3.1. Preparation of Samples
3.2. Protein Digestion
3.3. LC–Q–TOF–MS/MS Analysis
3.4. Protein and Peptide Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alexandratos, N.; Jorge, M. World Agriculture: Towards 2015/2030 An FAO Perspective. Available online: http://www.fao.org/docrep/005/y4252e/y4252e05b.htm (accessed on 17 December 2018).
- Brunner, T.A.; van der Horst, K.; Siegrist, M. Convenience food products. Drivers for consumption. Appetite 2010, 55, 498–506. [Google Scholar] [CrossRef]
- Shan, L.C.; De Brún, A.; Henchion, M.; Li, C.; Murrin, C.; Wall, P.G.; Monahan, F.J. Consumer evaluations of processed meat products reformulated to be healthier—A conjoint analysis study. Meat Sci. 2017, 131, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.M.; dos Santos Silva, V.L.; Trindade, M.A. Consumers’ perception of beef burgers with different healthy attributes. LWT Food Sci. Technol. 2014, 59, 1227–1232. [Google Scholar] [CrossRef]
- Castro-Rubio, F.; García, M.C.; Rodríguez, R.; Marina, M.L. Simple and Inexpensive Method for the Reliable Determination of Additions of Soybean Proteins in Heat-Processed Meat Products: An Alternative to the AOAC Official Method. J. Agric. Food Chem. 2005, 53, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.R.d.; Milani, T.M.G.; Trinca, N.R.R.; Nagai, L.Y.; Barretto, A.C.d.S. Textured soy protein, collagen and maltodextrin as extenders to improve the physicochemical and sensory properties of beef burger. Food Sci. Technol. 2017, 37, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Cassini, A.S.; Marczak, L.D.F.; Noreña, C.P.Z. Drying Characteristics of Textured Soy Protein: A Comparison between Three Different Products. Dry. Technol. 2007, 25, 2047–2054. [Google Scholar] [CrossRef]
- Official Journal of the European Union Regulation UE No 1169/2011. Off. J. Eur. Union 2011, 54.
- Hung, S.C.; Zayas, J.F. Functionality of Milk Proteins and Corn Germ Protein Flour in Comminuted Meat Products. J. Food Qual. 1992, 15, 139–152. [Google Scholar] [CrossRef]
- Darewicz, M.; Dziuba, B.; Minkiewicz, P.; Dziuba, J. The Preventive Potential of Milk and Colostrum Proteins and Protein Fragments. Food Rev. Int. 2011, 27, 357–388. [Google Scholar] [CrossRef]
- Arihara, K. Strategies for designing novel functional meat products. Meat Sci. 2006, 74, 219–229. [Google Scholar] [CrossRef]
- Rossini, K.; Noreña, C.P.Z.; Cladera-Olivera, F.; Brandelli, A. Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat. LWT Food Sci. Technol. 2009, 42, 862–867. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Duthie, G.; Campbell, F.; Bestwick, C.; Stephen, S.; Russell, W. Antioxidant effectiveness of vegetable powders on the lipid and protein oxidative stability of cooked Turkey meat patties: Implications for health. Nutrients 2013, 5, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; Hopia, A.; Kivikari, R.; Kahkonen, M. Use of natural food/plant extracts: Cloudberry (Rubus Chamaemorus), beetroot (Beta Vulgaris “Vulgaris”) or willow herb (Epilobium angustifolium) to reduce lipid oxidation of cooked pork patties. LWT Food Sci. Technol. 2005, 38, 363–370. [Google Scholar] [CrossRef]
- Račkauskiene, I.; Pukalskas, A.; Venskutonis, P.R.; Fiore, A.; Troise, A.D.; Fogliano, V. Effects of beetroot (Beta vulgaris) preparations on the Maillard reaction products in milk and meat-protein model systems. Food Res. Int. 2015, 70, 31–39. [Google Scholar] [CrossRef]
- Montowska, M.; Fornal, E. Detection of peptide markers of soy, milk and egg white allergenic proteins in poultry products by LC-Q-TOF-MS/MS. LWT Food Sci. Technol. 2018, 87, 310–317. [Google Scholar] [CrossRef]
- Heick, J.; Fischer, M.; Pöpping, B. First screening method for the simultaneous detection of seven allergens by liquid chromatography mass spectrometry. J. Chromatogr. A 2011, 1218, 938–943. [Google Scholar] [CrossRef]
- Hoffmann, B.; Münch, S.; Schwägele, F.; Neusüß, C.; Jira, W. A sensitive HPLC-MS/MS screening method for the simultaneous detection of lupine, pea, and soy proteins in meat products. Food Control 2017, 71, 200–209. [Google Scholar] [CrossRef]
- Sanchez-Monge, R.; Lopez-Torrejón, G.; Pascual, C.Y.; Varela, J.; Martin-Esteban, M.; Salcedo, G. Vicilin and convicilin are potential major allergens from pea. Clin. Exp. Allergy 2004, 34, 1747–1753. [Google Scholar] [CrossRef]
- Gomaa, A.; Boye, J. Impact of irradiation and thermal processing on the immunochemical detection of milk and egg allergens in foods. Food Res. Int. 2015, 74, 275–283. [Google Scholar] [CrossRef]
- Packer, V.G.; Melo, P.S.; Bergamaschi, K.B.; Selani, M.M.; Villanueva, N.D.M.; de Alencar, S.M.; Contreras-Castillo, C.J. Chemical characterization, antioxidant activity and application of beetroot and guava residue extracts on the preservation of cooked chicken meat. J. Food Sci. Technol. 2015, 52, 7409–7416. [Google Scholar] [CrossRef]
- Monaci, L.; Pilolli, R.; De Angelis, E.; Godula, M.; Visconti, A. Multi-allergen detection in food by micro high-performance liquid chromatography coupled to a dual cell linear ion trap mass spectrometry. J. Chromatogr. A 2014, 1358, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Montowska, M.; Fornal, E. Absolute quantification of targeted meat and allergenic protein additive peptide markers in meat products. Food Chem. 2019, 274, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, P.; Pi, F.; Sun, C.; Sun, J.; Jia, M.; Ying, C.; Zhang, Y.; Sun, X. Development of a liquid chromatography-tandem mass spectrometry method for simultaneous detection of the main milk allergens. Food Control 2017, 74, 79–88. [Google Scholar] [CrossRef]
- Chen, Q.; Ke, X.; Zhang, J.S.; Lai, S.Y.; Fang, F.; Mo, W.M.; Ren, Y.P. Proteomics method to quantify the percentage of cow, goat, and sheep milks in raw materials for dairy products. J. Dairy Sci. 2016, 99, 9483–9492. [Google Scholar] [CrossRef] [PubMed]
- Mallick, P.; Kuster, B. Proteomics: A pragmatic perspective. Nat. Biotechnol. 2010, 28, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Clerens, S.; Farouk, M.M. LC MS/MS identification of large structural proteins from bull muscle and their degradation products during post mortem storage. Food Chem. 2014, 150, 137–144. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Swiss-Prot Protein Knowledgebase, release 47.3 Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Ingredient (%) | Burger B1/B5 | Burger B2/B6 | Burger B3/B7 | Burger B4/B8 |
|---|---|---|---|---|
| Beef | 51 | 51 | 80 | 100 |
| Fat | ≤20 | ≤20 | ≤20 | ≤20 |
| Collagen | ≤25 | ≤25 | ≤25 | ≤15 |
| Proteins | 11.5 | 13.1 | 16.0 | 19.0 |
| NaCl | 0.95 | 1.1 | 0.8 | n.d.1 |
| Mix | M1 | M2 | M3 | |
| Non-meat proteins | Soy proteins rehydrated (30%) | Soy proteins rehydrated (24%) Milk proteins | Soy proteins Pea proteins Traces of milk proteins | n.d. |
| Other non-meat additives | Powdered onion 1.78%, water, sodium chloride, wheat fibre, plant flavours, breadcrumbs, monosodium glutamate, powdered beetroot juice, spices | Water, sodium chloride, wheat fibre, plant flavours, breadcrumbs, powdered beetroot juice, spices | Water, sodium chloride, plant flavours, powdered beetroot juice, dextrose | n.d. |
| Identified Protein | Accession No. | Mass (Da) | Matched Peptides 1 | Sequence Coverage (%) 2 | Matched Peptides | Sequence Coverage (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | B5 | B6 | B7 | B5 | B6 | B7 | |||
| Seed lipoxygenase-1 | P08170 | 94,595.8 | 46 | 38 | 44 | 74.2 | 63.1 | 68.6 | 19 | 23 | 7 | 36.4 | 43.7 | 11.6 |
| Seed lipoxygenase-2 | P09439 | 97,429.1 | 47 | 34 | 45 | 73.8 | 52.7 | 71.5 | 18 | 5 | 7 | 28.5 | 5.9 | 9.3 |
| Seed lipoxygenase-3 | P09186 | 97,155.8 | 53 | 40 | 46 | 61.9 | 60 | 64 | 22 | 27 | 15 | 41.8 | 48.8 | 26.7 |
| β-Conglycinin, α chain | P13916 | 70,577.1 | 39 | 32 | 36 | 58.8 | 57 | 57 | 28 | 28 | 25 | 54.4 | 53.3 | 51.5 |
| β-Conglycinin, α′ chain | P11827 | 74,609.2 | 27 | 20 | 29 | 44.7 | 37.8 | 48.6 | 19 | 15 | 16 | 33.9 | 28.4 | 29.5 |
| β-Conglycinin, β chain | P25974 | 50,608.1 | 29 | 27 | 28 | 68.1 | 66.9 | 68.1 | 18 | 21 | 14 | 59.9 | 61.2 | 48.9 |
| Glycinin | P04347 | 54,960.3 | 20 | 18 | 19 | 62 | 55.8 | 62 | 16 | 16 | 25 | 52.3 | 52.3 | 51.5 |
| Glycinin G1 | P04776 | 54,868.3 | 29 | 28 | 28 | 83 | 83 | 81.4 | 24 | 24 | 23 | 80.2 | 79.3 | 75.9 |
| Glycinin G2 | P04405 | 64,042.4 | 28 | 26 | 27 | 83.7 | 83.7 | 75.4 | 20 | 22 | 20 | 73.6 | 82.6 | 71.5 |
| Glycinin G3 | P11828 | 58,411.5 | 24 | 21 | 26 | 80 | 73.5 | 80 | 17 | 17 | 11 | 71.5 | 64.2 | 45.5 |
| Glycinin G4 | P02858 | 56,332.9 | 31 | 28 | 29 | 61.3 | 65.3 | 61.3 | 21 | 23 | 20 | 53.0 | 53.2 | 52.8 |
| Sucrose-binding protein | Q04672 | 60,920.9 | 24 | 18 | 26 | 56.1 | 45 | 58.9 | 16 | 18 | 13 | 41.4 | 47.5 | 29.3 |
| Trypsin inhibitor A | P01070 | 24,290.1 | 14 | 15 | 15 | 56.4 | 60.1 | 58.7 | 11 | 11 | 11 | 53.7 | 49 | 49.5 |
| Kunitz-type trypsin inhibitor KTI1 | P25272 | 22,830.8 | 9 | 8 | 11 | 47.7 | 36.9 | 54.1 | 5 | 4 | 4 | 34.4 | 23.6 | 23.6 |
| β-Amylase | P10538 | 56,484.1 | 14 | 10 | 14 | 39.1 | 30 | 35 | 3 | 3 | 1 | 10.2 | 10.2 | 5 |
| Lectin | P05046 | 30,927.4 | 10 | 11 | 10 | 71.9 | 72.9 | 71.9 | 8 | 8 | 5 | 60.7 | 64.2 | 35 |
| 2S albumin | P19594 | 19,030.2 | 8 | 7 | 7 | 41.7 | 41.7 | 41.7 | 6 | 6 | 5 | 41.1 | 41.1 | 32.9 |
| P24 oleosin isoform B | P29531 | 23,392.2 | 7 | 7 | 7 | 30.4 | 30.4 | 30.4 | 4 | 3 | 3 | 18.8 | 13 | 13 |
| P34 probable thiol protease | P22895 | 43,135.4 | 6 | 6 | 6 | 22.1 | 22.1 | 22.1 | 5 | 5 | 4 | 21.8 | 21.8 | 21.6 |
| Basic 7S globulin | P13917 | 47,133.9 | 14 | 13 | 14 | 57.1 | 54.5 | 57.1 | 12 | 13 | 7 | 50.1 | 51.7 | 29.9 |
| Parent Ion (m/z) | Mr (exp) | Exp z 1 | Peptide Score 2 | Total Intensity Range | Peptide Marker | Protein | Protein Score 3 |
|---|---|---|---|---|---|---|---|
| 686.8540 | 1372.7002 | 2 | 19.99 | 1.23 × 106–1.01 × 108 | (R)ALIQVVNCNGER(V) | 558.84 | |
| 765.3553 | 1529.7013 | 2 | 18.57 | 3.05 × 106–7.95 × 107 | (R)EQPQQNECQIQK(L) | ||
| 586.8197 | 1172.6310 | 2 | 20.15 | 1.87 × 107–5.76 × 108 | (K)FLVPPQESQK(R) | Glycinin G1 | |
| 889.7510 | 2667.2330 | 3 | 22.57 | 1.84 × 107–8.57 × 108 | (K)GIFGMIYPGCPSTFEEPQQPQQR(G) | ||
| 820.1402 | 3277.5284 | 4 | 22.42 | 3.19 × 107–9.40 × 108 | (K)HQQEEENEGGSILSGFTLEFLEHAFSVDK(Q) | ||
| 554.3123 | 1107.6157 | 2 | 19.15 | 2.14 × 105–1.10 × 107 | (R)LSAEFGSLR(N) | ||
| 616.7735 | 1232.5390 | 2 | 16.95 | 1.22 × 106–3.56 × 107 | (K)NLQGENEGEDK(G) | ||
| 1141.5955 | 3422.7624 | 3 | 22.17 | 1.76 × 107–1.60 × 109 | (K)TNDTPMIGTLAGANSLLNALPEEVIQHTFNLK(S) | ||
| 575.2810 | 1149.5535 | 2 | 21.65 | 3.21 × 106–2.30 × 108 | (R)VFDGELQEGR(V) | ||
| 713.4353 | 1425.8576 | 2 | 24.28 | 2.64 × 107–4.45 × 108 | (R)VLIVPQNFVVAAR(S) | ||
| 709.3268 | 1417.6455 | 2 | 19.51 | 1.15 × 105–1.90 × 107 | (K)YQQEQGGHQSQK(G) | ||
| 793.8984 | 1586.7849 | 2 | 23.48 | 1.53 × 107–3.68 × 108 | (R)FYLAGNQEQEFLK(Y) | Glycinin G1/G2 | 558.84/549.21 |
| 725.8287 | 1450.6485 | 2 | 21.39 | 1.94 × 107–3.82 × 108 | (R)SQSDNFEYVSFK(T) | G1–G3 | 558/549/449 |
| 1246.6297 | 2492.2503 | 2 | 22.62 | 9.73 × 105–1.61 × 108 | (K)NAMFVPHYNLNANSIIYALNGR(A) | Glycinin G1/G3 | 558.84/449.67 |
| 679.8461 | 1358.6845 | 2 | 19.95 | 6.93 × 105–6.69 × 107 | (R)ALVQVVNCNGER(V) | ||
| 632.3305 | 1263.6514 | 2 | 19.95 | 1.33 × 106–3.23 × 108 | (K)EAFGVNMQIVR(N) | Glycinin G2 | 549.21 |
| 897.7005 | 2691.0846 | 3 | 19.95 | 4.33 × 105–1.16 × 108 | (R)KPQQEEDDDDEEEQPQCVETDK(G) | ||
| 497.7697 | 994.5316 | 2 | 18.63 | 4.55 × 106–8.60 × 107 | (K)LSAQYGSLR(K) | ||
| 1240.1321 | 2479.2551 | 2 | 20.70 | 1.54 × 106–1.56 × 108 | (K)NAMFVPHYTLNANSIIYALNGR(A) | ||
| 966.4660 | 1931.9193 | 2 | 24.50 | 1.12 × 107–6.50 × 108 | (R)NLQGENEEEDSGAIVTVK(G) | ||
| 937.4649 | 1873.9191 | 2 | 20.23 | 1.11 × 107–6.50 × 108 | (K)NNNPFSFLVPPQESQR(R) | ||
| 915.9747 | 3660.8544 | 3 | 19.69 | 1.03 × 107–4.26 × 108 | (R)QNIGQNSSPDIYNPQAGSITTATSLDFPALWLLK(L) | ||
| 1183.5625 | 2366.1147 | 2 | 22.92 | 1.41 × 107–5.19 × 108 | (K)QQEEENEGSNILSGFAPEFLK(E) | ||
| 1201.1432 | 2401.2762 | 2 | 22.88 | 3.55 × 107–9.17 × 108 | (R)VFDGELQEGGVLIVPQNFAVAAK(S) | Glycinin G2 | |
| 747.8511 | 1494.6932 | 2 | 17.89 | 3.91 × 105–3.49 × 107 | (K)YQQQQQGGSQSQK(G) | 549.21 | |
| 1116.0425 | 2231.0768 | 2 | 20.69 | 4.61 × 105–7.80 × 107 | (R)FYLAGNQEQEFLQYQPQK(Q) | Glycinin G3 | 449.67 |
| 1205.6108 | 3614.8126 | 3 | 20.39 | 3.05 × 106–2.46 × 108 | (R)HNIGQTSSPDIFNPQAGSITTATSLDFPALSWLK(L) | ||
| 1045.4962 | 3134.4701 | 3 | 24.92 | 8.48 × 105–1.43 × 108 | (R)QQEEENEGGSILSGFAPEFLEHAFVVDR(Q) | ||
| 867.2119 | 3465.8085 | 4 | 19.47 | 1.11 × 106–2.79 × 107 | (K)TNDRPSIGNLAGANSLLNALPEEVIQQTFNLR(R) | ||
| 1047.1164 | 2093.2216 | 2 | 22.67 | 3.90 × 107–2.59 × 108 | (K)AIVILVINEGDANIELVGLK(K) | β-Conglycinin, α chain | 675.52 |
| 901.1371 | 2701.3906 | 3 | 22.17 | 1.31 × 107–3.61 × 108 | (R)DLDIFLSIVDMNEGALLLPHFNSK(A) | ||
| 1013.4783 | 2025.9472 | 2 | 23.37 | 3.79 × 106–1.48 × 108 | (K)EQQQEQQQEEQPLEVR(K) | ||
| 592.2857 | 1183.5630 | 2 | 16.74 | 2.38 × 106–1.38 × 108 | (R)ESYFVDAQPK(K) | ||
| 1124.9099 | 3372.7070 | 3 | 19.96 | 2.48 × 107–1.45 × 109 | (R)NFLAGSQDNVISQIPSQVQELAFPGSAQAVEK(L) | ||
| 526.2697 | 1051.5320 | 2 | 14.05 | 2.03 × 106–2.36 × 108 | (K)NPFLFGSNR(F) | ||
| 1076.5199 | 2152.0305 | 2 | 23.11 | 2.01 × 107–5.28 × 108 | (R)VPSGTTYYVVNPDNNENLR(L) | ||
| 622.8594 | 1244.7096 | 2 | 20.04 | 1.31 × 107–2.47 × 108 | (R)LQESVIVEISK(E) | β-Conglycinin, α/α’ chain | 675.52/482.98 |
| 577.2909 | 1153.5735 | 2 | 18.62 | 5.51 × 106–1.82 × 108 | (R)NILEASYDTK(F) | ||
| 698.4148 | 2093.2216 | 3 | 18.22 | 7.53 × 106–4.52 × 107 | (K)AIVVLVINEGEANIELVGIK(E) | β-Conglycinin, α’ chain | 482.98 |
| 903.1223 | 2707.3436 | 3 | 21.01 | 5.11 × 106–3.15 × 108 | (R)DLDVFLSVVDMNEGALFLPHFNSK(A) | ||
| 669.8188 | 1338.6284 | 2 | 18.65 | 9.42 × 105–1.41 × 107 | (R)DSYNLQSGDALR(V) | ||
| 1215.5866 | 2430.1612 | 2 | 21.58 | 4.20 × 105–1.29 × 108 | (R)FESFFLSSTQAQQSYLQGFSK(N) | ||
| 1047.1164 | 2093.2216 | 2 | 22.67 | 3.90 × 107–2.59 × 108 | (K)AIVILVINEGDANIELVGIK(E) | β-Conglycinin, β chain | 546.79 |
| 886.4683 | 2657.3821 | 3 | 22.61 | 6.34 × 106–2.00 × 108 | (R)DLDIFLSSVDINEGALLLPHFNSK(A) | ||
| 615.3408 | 1229.6736 | 2 | 16.74 | 1.54 × 106–1.16 × 108 | (R)QQEGVIVELSK(E) | ||
| 887.4435 | 1773.8766 | 2 | 18.78 | 1.79 × 106–1.12 × 108 | (R)QVQELAFPGSAQDVER(L) | ||
| 478.7618 | 956.5160 | 2 | 17.37 | 1.40 × 106–5.29 × 107 | (R)SPQLENLR(D) |
| Parent Ion (m/z) | Mr (exp) | Exp z | Peptide Score | Total Intensity Range | Peptide Marker | Protein | Protein Score |
|---|---|---|---|---|---|---|---|
| Milk Peptides | |||||||
| 858.4109 | 1715.8057 | 2 | 20.69 | 8.80 × 106–6.91 × 108 | (R)LSFNPTQLEEQCHI(-) | β-Lactoglobulin | 245.95 |
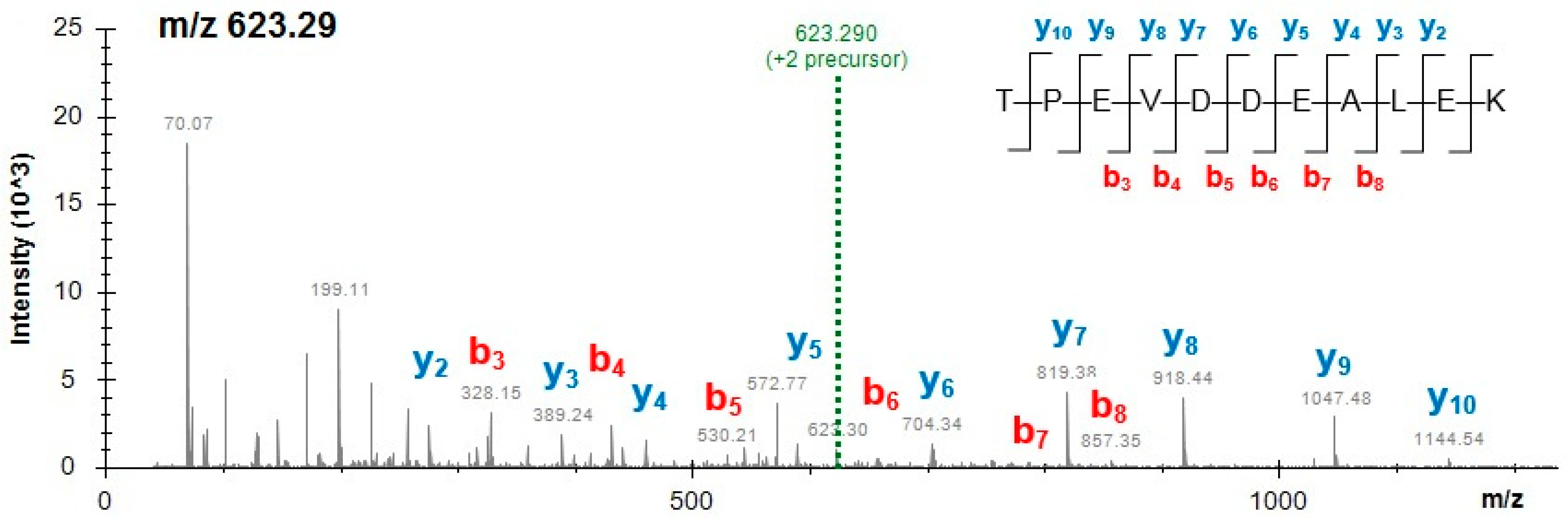
| 623.2994 | 1245.5845 | 2 | 22.62 | 4.95 × 107–1.49 × 108 | (R)TPEVDDEALEK(F) | ||
| 772.7189 | 2316.1369 | 3 | 18.97 | 3.77 × 107–4.27 × 107 | (K)EPMIGVNQELAYFYPELFR(Q) | αS1-Casein | 106.74 |
| 692.8696 | 1384.7300 | 2 | 18.97 | 4.15 × 107–4.61 × 107 | (R)FFVAPFPEVFGK(E) | ||
| 880.4773 | 1759.9450 | 2 | 20.53 | 4.35 × 107–4.82 × 107 | (K)HQGLPQEVLNENLLR(F) | ||
| 374.2062 | 747.4036 | 2 | 10.70 | 3.30 × 105–7.24 × 105 | (R)APVDAFK(E) | Lactotransferrin | 56.56 |
| 638.8128 | 1276.6168 | 2 | 17.53 | 1.09 × 106–1.19 × 106 | (R)NPDEEGLFTVR(A) | Butyrophilin subfamily 1 member A1 | 80.89 |
| 481.2771 | 961.5465 | 2 | 16.66 | 1.11 × 106–2.21 × 106 | (K)VSPAVFVSR(E) | ||
| 854.9494 | 1708.8905 | 2 | 14.72 | 9.73 × 105–2.10 × 106 | (K)INLFDTPLETQYVR(L) | Lactadherin | 40.87 |
| 374.2060 | 1120.6010 | 3 | 14.82 | 4.33 × 105–7.64 × 105 | (R)IQPVAWHNR(I) | ||
| 990.5502 | 1980.0913 | 2 | 16.02 | 6.20 × 106–1.21 × 107 | (R)SPAQILQWQVLSNTVPAK(S) | κ-Casein | 34.99 |
| 630.8260 | 1260.6430 | 2 | 14.40 | 3.86 × 106–4.31 × 106 | (R)NLQISNEDLSK(E) | Glycosylation-dependent cell adhesion molecule 1 | 43.40 |
| 421.8969 | 1263.6732 | 3 | 16.35 | 1.23 × 107–1.32 × 107 | (K)SLFSHAFEVVK(T) | ||
| Pea Peptides | |||||||
| 545.2826 | 1633.8293 | 3 | 12.27 | 5.19 × 105–9.63 × 105 | (K)VLLEQQEQEPQHR(R) | Provicilin (Fragment) | 59.72 |
| 957.9525 | 1914.8967 | 2 | 12.61 | 1.06 × 106–1.16 × 106 | (K)NILEASFNTDYEEIEK(V) | Vicilin | 47.03 |
| Beetroot Peptides | |||||||
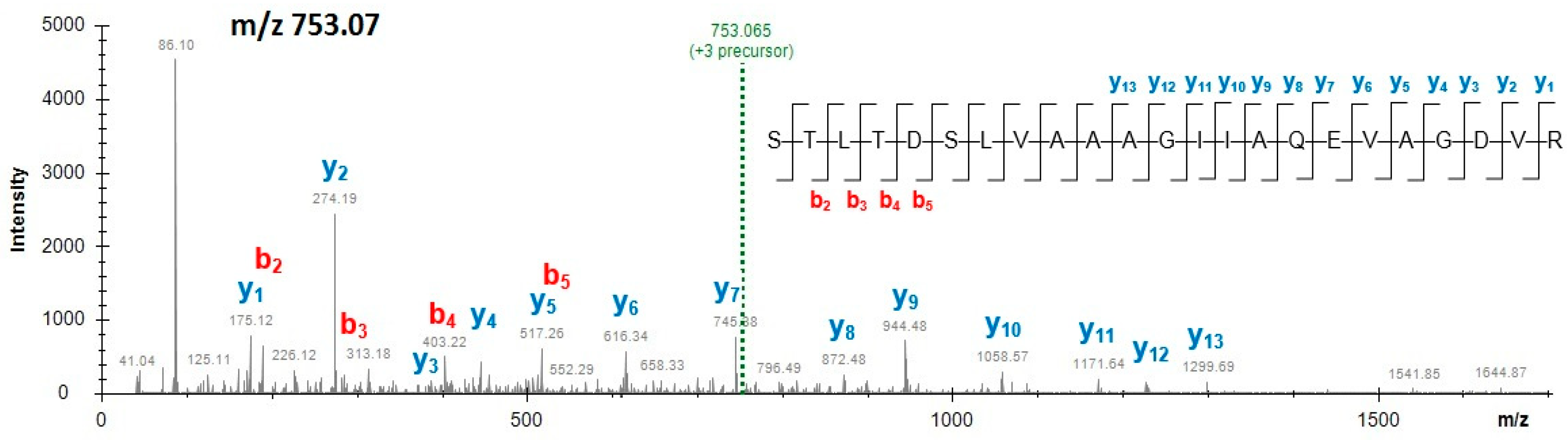
| 753.0750 | 2257.2034 | 3 | 21.82 | 1.09 × 106–9.85 × 106 | (K)STLTDSLVAAAGIIAQEVAGDVR(M) | Elongation factor 2 (EF-2) | 88.19 |
| 974.1526 | 2920.4649 | 3 | 15.64 | 2.03 × 106–2.11 × 106 | (K)VIENANVIMATYEDPLLGDVQVYPEK(G) | ||
| 560.2594 | 1119.5099 | 2 | 14.72 | 2.90 × 105–7.04 × 105 | (K)EGALAEENMR(G) | ||
| 372.6955 | 744.3828 | 2 | 11.03 | 8.55 × 105–1.69 × 106 | (R)FFAFGR(V) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikołajczak, B.; Fornal, E.; Montowska, M. LC–Q–TOF–MS/MS Identification of Specific Non-Meat Proteins and Peptides in Beef Burgers. Molecules 2019, 24, 18. https://doi.org/10.3390/molecules24010018
Mikołajczak B, Fornal E, Montowska M. LC–Q–TOF–MS/MS Identification of Specific Non-Meat Proteins and Peptides in Beef Burgers. Molecules. 2019; 24(1):18. https://doi.org/10.3390/molecules24010018
Chicago/Turabian StyleMikołajczak, Beata, Emilia Fornal, and Magdalena Montowska. 2019. "LC–Q–TOF–MS/MS Identification of Specific Non-Meat Proteins and Peptides in Beef Burgers" Molecules 24, no. 1: 18. https://doi.org/10.3390/molecules24010018






