The Blossoming of Technology for the Analysis of Complex Aroma Bouquets—A Review on Flavour and Odorant Multidimensional and Comprehensive Gas Chromatography Applications
Abstract
:1. Introduction
2. Food
2.1. Fruits and Nuts
2.2. Honey
2.3. Dairy Products
2.4. Flour and Pasta
2.5. Meats and Seafood
2.6. Seasonings and Related Samples
3. Drinks and Beverages
4. Essential Oils and Fragrances
5. Wood
6. Incense
7. Tobacco and Cigarette
8. Environmental
9. Biologics
10. Forensics
11. Plastic Products Applications
12. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Barcelo, D.; Ramos, L. Comprehensive Two Dimensional Gas Chromatography; Elsevier: Amsterdam, The Netherlands, 2009; Volume 55, p. 305. [Google Scholar]
- Mondello, L.; Bartle, K.; Lewis, A. Multidimensional Chromatography; OMICS International: Hyderabad, India, 2001; p. 448. [Google Scholar]
- Prebihalo, S.E.; Berrier, K.L.; Freye, C.E.; Bahaghighat, H.D.; Moore, N.R.; Pinkerton, D.K.; Synovec, R.E. Multidimensional Gas Chromatography: Advances in Instrumentation, Chemometrics, and Applications. Analy. Chem. 2018, 90, 505–532. [Google Scholar] [CrossRef]
- Cordero, C.; Kiefl, J.; Reichenbach, S.E.; Bicchi, C. Characterization of odorant patterns by comprehensive two-dimensional gas chromatography: A challenge in omic studies. TrAC Trends Analy. Chem. 2018. In press. [Google Scholar] [CrossRef]
- Cordero, C.; Schmarr, H.-G.; Reichenbach, S.E.; Bicchi, C. Current Developments in Analyzing Food Volatiles by Multidimensional Gas Chromatographic Techniques. J. Agric. Food Chem. 2018, 66, 2226–2236. [Google Scholar] [CrossRef]
- Chin, S.-T.; Marriott, P.J. Review of the role and methodology of high resolution approaches in aroma analysis. Analy. Chim. Acta 2015, 854, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Gas Chromatography-Mass Spectrometry in Odorant Analysis, 1st ed.; Springer International Publishing: New York, NY, USA, 2017; p. 1151. [Google Scholar]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. System Design for Integrated Comprehensive and Multidimensional Gas Chromatography with Mass Spectrometry and Olfactometry. Analy. Chem. 2012, 84, 9154–9162. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Application of integrated comprehensive/multidimensional gas chromatography with mass spectrometry and olfactometry for aroma analysis in wine and coffee. Food Chem. 2015, 185, 355–361. [Google Scholar] [CrossRef]
- Mitrevski, B.; Marriott, P.J. Novel Hybrid Comprehensive 2D-Multidimensional Gas Chromatography for Precise, High-Resolution Characterization of Multicomponent Samples. Analy. Chem. 2012, 84, 4837–4843. [Google Scholar] [CrossRef]
- Maikhunthod, B.; Morrison, P.D.; Small, D.M.; Marriott, P.J. Development of a switchable multidimensional/comprehensive two-dimensional gas chromatographic analytical system. J. Chrom. A 2010, 1217, 1522–1529. [Google Scholar] [CrossRef]
- Yan, D.; Wong, Y.F.; Whittock, S.P.; Koutoulis, A.; Shellie, R.A.; Marriott, P.J. Sequential Hybrid Three-Dimensional Gas Chromatography with Accurate Mass Spectrometry: A Novel Tool for High-Resolution Characterization of Multicomponent Samples. Analy. Chem. 2018, 90, 5264–5271. [Google Scholar] [CrossRef]
- Zeng, A.X.; Chin, S.-T.; Marriott, P.J. Integrated multidimensional and comprehensive 2D GC analysis of fatty acid methyl esters. J. Sep. Sci. 2013, 36, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N. Selectable one-dimensional or two-dimensional gas chromatography-mass spectrometry. In Flavor, Fragrance, and Odor Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 21–46. [Google Scholar]
- Ochiai, N.; Sasamoto, K. Selectable one-dimensional or two-dimensional gas chromatography-olfactometry/mass spectrometry with preparative fraction collection for analysis of ultra-trace amounts of odor compounds. J. Chrom. A 2011, 1218, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Adahchour, M.; Jover, E.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Twin comprehensive two-dimensional gas chromatographic system: Concept and applications. J. Chrom. A 2005, 1086, 128–134. [Google Scholar] [CrossRef]
- Begnaud, F.; Debonneville, C.; Probst, J.-P.; Chaintreau, A.; Morrison, P.D.; Adcock, J.L.; Marriott, P.J. Effects of variation in modulator temperature during cryogenic modulation in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2009, 32, 3144–3151. [Google Scholar] [CrossRef]
- Chin, S.-T.; Maikhunthod, B.; Marriott, P.J. Universal Method for Online Enrichment of Target Compounds in Capillary Gas Chromatography Using In-Oven Cryotrapping. Analy. Chem. 2011, 83, 6485–6492. [Google Scholar] [CrossRef]
- Ruehle, C.; Eyres, G.T.; Urban, S.; Dufour, J.-P.; Morrison, P.D.; Marriott, P.J. Multiple component isolation in preparative multidimensional gas chromatography with characterization by mass spectrometry and nuclear magnetic resonance spectroscopy. J. Chrom. A 2009, 1216, 5740–5747. [Google Scholar] [CrossRef]
- Yang, S.-O.; Kim, Y.; Kim, H.-s.; Hyun, S.-H.; Kim, S.-H.; Choi, H.-K.; Marriott, P.J. Rapid sequential separation of essential oil compounds using continuous heart-cut multi-dimensional gas chromatography-mass spectrometry. J. Chrom. A 2011, 1218, 2626–2634. [Google Scholar] [CrossRef]
- Mastello, R.B.; Capobiango, M.; Chin, S.-T.; Monteiro, M.; Marriott, P.J. Identification of odour-active compounds of pasteurised orange juice using multidimensional gas chromatography techniques. Food Res. Int. 2015, 75, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubinska-Szczygel, M.; Rozanska, A.; Dymerski, T.; Namiesnik, J.; Katrich, E.; Gorinstein, S. A novel analytical approach in the assessment of unprocessed Kaffir lime peel and pulp as potential raw materials for cosmetic applications. Ind. Crops Prod. 2018, 120, 313–321. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frerot, E. Comparative analysis of three Australian finger lime (Citrus australasica) cultivars: Identification of unique citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef]
- Hong, J.H.; Khan, N.; Jamila, N.; Hong, Y.S.; Nho, E.Y.; Choi, J.Y.; Lee, C.M.; Kim, K.S. Determination of Volatile Flavour Profiles of Citrus spp. Fruits by SDE-GC-MS and Enantiomeric Composition of Chiral Compounds by MDGC-MS. Phytochem. Analy. 2017, 28, 392–403. [Google Scholar] [CrossRef]
- Casilli, A.; Decorzant, E.; Jaquier, A.; Delort, E. Multidimensional gas chromatography hyphenated to mass spectrometry and olfactometry for the volatile analysis of citrus hybrid peel extract. J. Chrom. A 2014, 1373, 169–178. [Google Scholar] [CrossRef]
- Steingass, C.B.; Carle, R.; Schmarr, H.-G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: I. Characterization of pineapple aroma compounds by comprehensive two-dimensional gas chromatography-mass spectrometry. Analy. BioAnaly. Chem. 2015, 407, 2591–2608. [Google Scholar] [CrossRef]
- Steingass, C.B.; Jutzi, M.; Mueller, J.; Carle, R.; Schmarr, H.-G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: II. Multivariate statistical profiling of pineapple aroma compounds based on comprehensive two-dimensional gas chromatography-mass spectrometry. Analy. BioAnaly. Chem. 2015, 407, 2609–2624. [Google Scholar] [CrossRef]
- Werkhoff, P.; Guentert, M.; Krammer, G.; Sommer, H.; Kaulen, J. Vacuum headspace method in aroma research: Flavor chemistry of yellow passion fruits. J. Agric. Food Chem. 1998, 46, 1076–1093. [Google Scholar] [CrossRef]
- Strohalm, H.; Dregus, M.; Wahl, A.; Engel, K.-H. Enantioselective Analysis of Secondary Alcohols and Their Esters in Purple and Yellow Passion Fruits. J. Agric. Food Chem. 2007, 55, 10339–10344. [Google Scholar] [CrossRef] [PubMed]
- Samykanno, K.; Pang, E.; Marriott, P.J. Chemical characterisation of two Australian-grown strawberry varieties by using comprehensive two-dimensional gas chromatography-mass spectrometry. Food Chem. 2013, 141, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.J.; Agyemang, D.; Curto, N.L.; Yusuf, A.; Chen, M.Z.; Janczuk, A.J. In-depth analysis of Ciflorette strawberries (Fragaria × ananassa ‘Ciflorette’) by multidimensional gas chromatography and gas chromatography-olfactometry. Flavour Fragr. J. 2015, 30, 302–319. [Google Scholar] [CrossRef]
- Sewenig, S.; Bullinger, D.; Hener, U.; Mosandl, A. Comprehensive authentication of (E)-α(β)-ionone from raspberries, using constant flow MDGC-C/P-IRMS and enantio-MDGC-MS. J. Agric. Food Chem. 2005, 53, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Kupska, M.; Wardencki, W.; Namiesnik, J. Application of response surface methodology to optimize solid-phase microextraction procedure for chromatographic determination of aroma-active monoterpenes in berries. Food Chem. 2017, 221, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Magagna, F.; Liberto, E.; Reichenbach, S.E.; Tao, Q.; Carretta, A.; Cobelli, L.; Giardina, M.; Bicchi, C.; Cordero, C. Advanced fingerprinting of high-quality cocoa: Challenges in transferring methods from thermal to differential-flow modulated comprehensive two dimensional gas chromatography. J. Chrom. A 2018, 1536, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.C.; Liberto, E.; Spigolon, N.; Fontana, M.; Somenzi, M.; Bicchi, C.; Cordero, C. Evolution of potent odorants within the volatile metabolome of high-quality hazelnuts (Corylus avellana L.): Evaluation by comprehensive two-dimensional gas chromatography coupled with mass spectrometry. Analy. BioAnaly. Chem. 2018, 410, 3491–3506. [Google Scholar] [CrossRef] [PubMed]
- Kiefl, J.; Pollner, G.; Schieberle, P. Sensomics analysis of key hazelnut odorants (Corylus avellana L. ‘Tonda Gentile’) using comprehensive two-dimensional gas chromatography in combination with time-of-flight mass spectrometry (GC×GC-TOF-MS). J. Agric. Food Chem. 2013, 61, 5226–5235. [Google Scholar] [CrossRef] [PubMed]
- Kiefl, J.; Schieberle, P. Evaluation of process parameters governing the aroma generation in three hazelnut cultivars (Corylus avellana L.) by correlating quantitative key odorant profiling with sensory evaluation. J. Agric. Food Chem. 2013, 61, 5236–5244. [Google Scholar] [CrossRef]
- Siegmund, B.; Urdl, K.; Jurek, A.; Leitner, E. “More than Honey”: Investigation on Volatiles from Monovarietal Honeys Using New Analytical and Sensory Approaches. J. Agric. Food Chem. 2018, 66, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Adahchour, M.; Wiewel, J.; Verdel, R.; Vreuls, R.J.J.; Brinkman, U.A.T. Improved determination of flavour compounds in butter by solid-phase (micro)extraction and comprehensive two-dimensional gas chromatography. J. Chrom. A 2005, 1086, 99–106. [Google Scholar] [CrossRef]
- Schuett, J.; Schieberle, P. Quantitation of Nine Lactones in Dairy Cream by Stable Isotope Dilution Assays Based on Novel Syntheses of Carbon-13-Labeled γ-Lactones and Deuterium-Labeled δ-Lactones in Combination with Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 10534–10541. [Google Scholar]
- Costa, R.; Albergamo, A.; Bua, G.D.; Saija, E.; Dugo, G. Determination of flavor constituents in particular types of flour and derived pasta by heart-cutting multidimensional gas chromatography coupled with mass spectrometry and multiple headspace solid-phase microextraction. LWT Food Sci. Technol. 2017, 86, 99–107. [Google Scholar] [CrossRef]
- Chaintreau, A.; Rochat, S.; de Saint Laumer, J.-Y. Re-investigation of sulfur impact odorants in roast beef using comprehensive two-dimensional GC-TOF-MS and the GC-SNIF. Develop. Food Sci. 2006, 43, 601–604. [Google Scholar]
- Rochat, S.; De Saint Laumer, J.-Y.; Chaintreau, A. Analysis of sulfur compounds from the in-oven roast beef aroma by comprehensive two-dimensional gas chromatography. J. Chrom. A 2007, 1147, 85–94. [Google Scholar] [CrossRef]
- Bueno, M.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. A model explaining and predicting lamb flavour from the aroma-active chemical compounds released upon grilling light lamb loins. Meat Sci. 2014, 98, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Mercier, F.; Tournayre, P.; Martin, J.-L.; Berdague, J.-L. Identification and origin of odorous sulfur compounds in cooked ham. Food Chem. 2014, 155, 207–213. [Google Scholar] [CrossRef]
- Wang, W.; Feng, X.; Zhang, D.; Li, B.; Sun, B.; Tian, H.; Liu, Y. Analysis of volatile compounds in Chinese dry-cured hams by comprehensive two-dimensional gas chromatography with high-resolution time-of-flight mass spectrometry. Meat Sci. 2018, 140, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Rochat, S.; Egger, J.; Chaintreau, A. Strategy for the identification of key odorants: Application to shrimp aroma. J. Chrom. A 2009, 1216, 6424–6432. [Google Scholar] [CrossRef] [PubMed]
- Leduc, F.; Tournayre, P.; Kondjoyan, N.; Mercier, F.; Malle, P.; Kol, O.; Berdague, J.L.; Duflos, G. Evolution of volatile odorous compounds during the storage of European seabass (Dicentrarchus labrax). Food Chem. 2012, 131, 1304–1311. [Google Scholar] [CrossRef]
- Huang, X.-H.; Zheng, X.; Chen, Z.-H.; Zhang, Y.-Y.; Du, M.; Dong, X.-P.; Qin, L.; Zhu, B.-W. Fresh and grilled eel volatile fingerprinting by e-Nose, GC-O, GC-MS and GC × GC-QTOF combined with purge and trap and solvent-assisted flavor evaporation. Food Res. Int. 2018. In press. [Google Scholar] [CrossRef] [PubMed]
- Eyres, G.; Dufour, J.-P.; Hallifax, G.; Sotheeswaran, S.; Marriott, P.J. Identification of character-impact odorants in coriander and wild coriander leaves using gas chromatography-olfactometry (GCO) and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC × GC-TOFMS). J. Sep. Sci. 2005, 28, 1061–1074. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.-P.; Hallifax, G.; Sotheeswaran, S.; Marriott, P.J. Identification of character impact odorants in coriander and wild coriander leaves using GC-olfactometry and GC x GC-TOFMS. Develop. Food Sci. 2006, 43, 197–200. [Google Scholar]
- Eyres, G.; Marriott, P.J.; Dufour, J.-P. The combination of gas chromatography-olfactometry and multidimensional gas chromatography for the characterization of essential oils. J. Chrom. A 2007, 1150, 70–77. [Google Scholar] [CrossRef]
- Steinhaus, M. Confirmation of 1-Phenylethane-1-thiol as the Character Impact Aroma Compound in Curry Leaves and Its Behavior during Tissue Disruption, Drying, and Frying. J. Agric. Food Chem. 2017, 65, 2141–2146. [Google Scholar] [CrossRef]
- Maikhunthod, B.; Marriott, P.J. Aroma-impact compounds in dried spice as a quality index using solid phase microextraction with olfactometry and comprehensive two-dimensional gas chromatography. Food Chem. 2013, 141, 4324–4332. [Google Scholar] [CrossRef]
- Shao, Y.; Marriott, P.; Shellie, R.; Hugel, H. Solid-phase micro-extraction-comprehensive two-dimensional gas chromatography of ginger (Zingiber officinale) volatiles. Flavour Fragr. J. 2003, 18, 5–12. [Google Scholar] [CrossRef]
- Lukic, I.; Carlin, S.; Horvat, I.; Vrhovsek, U. Combined targeted and untargeted profiling of volatile aroma compounds with comprehensive two-dimensional gas chromatography for differentiation of virgin olive oils according to variety and geographical origin. Food Chem. 2019, 270, 403–414. [Google Scholar] [CrossRef]
- Sghaier, L.; Cordella, C.B.Y.; Rutledge, D.N.; Watiez, M.; Breton, S.; Kopczuk, A.; Sassiat, P.; Thiebaut, D.; Vial, J. Comprehensive Two-dimensional Gas Chromatography for Analysis of the Volatile Compounds and Fishy Odor Off-flavors from Heated Rapeseed Oil. Chromatographia 2015, 78, 805–817. [Google Scholar] [CrossRef]
- Gracka, A.; Raczyk, M.; Hradecky, J.; Hajslova, J.; Jeziorski, S.; Karlovits, G.; Michalak, B.; Bakowska, N.; Jelen, H. Volatile compounds and other indicators of quality for cold-pressed rapeseed oils obtained from peeled, whole, flaked and roasted seeds. Euro. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, S.; Kong, X.; Ji, Z.; Han, X.; Wu, J.; Mao, J. Elucidation of the aroma compositions of Zhenjiang aromatic vinegar using comprehensive two dimensional gas chromatography coupled to time-of-flight mass spectrometry and gas chromatography-olfactometry. J. Chrom. A 2017, 1487, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Koutidou, M.; Grauwet, T.; Van Loey, A.; Acharya, P. Impact of processing on odour-active compounds of a mixed tomato-onion puree. Food Chem. 2017, 228, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Sciarrone, D.; Schepis, A.; Zoccali, M.; Donato, P.; Vita, F.; Creti, D.; Alpi, A.; Mondello, L. Multidimensional Gas Chromatography Coupled to Combustion-Isotope Ratio Mass Spectrometry/Quadrupole MS with a Low-Bleed Ionic Liquid Secondary Column for the Authentication of Truffles and Products Containing Truffle. Analy. Chem. 2018, 90, 6610–6617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Sun, B.; Mao, X.; Zhang, Y.; Zhou, Y. Comparative analysis of volatile composition in Chinese truffles via GC × GC/HR-TOF/MS and electronic nose. Int. J. Mol. Sci. 2016, 17, 412. [Google Scholar] [CrossRef]
- Yuan, F.; He, F.; Qian, Y.; Zheng, J.; Qian, M.C. Aroma Stability of Lemon-Flavored Hard Iced Tea Assessed by Chirality and Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2016, 64, 5717–5723. [Google Scholar] [CrossRef]
- He, F.; Qian, Y.P.L.; Qian, M.C. Flavor and chiral stability of lemon-flavored hard tea during storage. Food Chem. 2018, 239, 622–630. [Google Scholar] [CrossRef]
- Kfoury, N.; Baydakov, E.; Gankin, Y.; Robbat, A., Jr. Differentiation of key biomarkers in tea infusions using a target/nontarget gas chromatography/mass spectrometry workflow. Food Res. Int. 2018, 113, 414–423. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.-P.; Shao, C.-Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.-D.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, K.; Ochiai, N. Selectable one-dimensional or two-dimensional gas chromatography-mass spectrometry with simultaneous olfactometry or element-specific detection. J. Chrom. A 2010, 1217, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Identification of potent odourants in wine and brewed coffee using gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. J. Chrom. A 2011, 1218, 7487–7498. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Hug, C.; Baggenstoss, J.; Blank, I.; Kerler, J. Emerging analytical techniques for the assessment of aroma relevant sulfur compounds in coffee. ACS Symposium Series 2011, 1068, 77–92. [Google Scholar]
- Majcher, M.A.; Klensporf-Pawlik, D.; Dziadas, M.; Jelen, H.H. Identification of aroma active compounds of cereal coffee brew and its roasted ingredients. J. Agric. Food Chem. 2013, 61, 2648–2654. [Google Scholar] [CrossRef]
- Miyazato, H.; Nakamura, M.; Hashimoto, S.; Hayashi, S. Identification of the odour-active cyclic diketone cis-2,6-dimethyl-1,4-cyclohexanedione in roasted Arabica coffee brew. Food Chem. 2013, 138, 2346–2355. [Google Scholar] [CrossRef]
- Miyazato, H.; Nakamura, M.; Hashimoto, S.; Hayashi, S. Odor-active (E)-4-methyl-3-hexenoic acid in roasted coffee generated in the Maillard reactions of l-isoleucine with sugars. Adv. J. Food Sci. Technol. 2013, 5, 1367–1374. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. Solid phase extraction, multidimensional gas chromatography mass spectrometry determination of four novel aroma powerful ethyl esters. J. Chrom. A 2007, 1140, 180–188. [Google Scholar] [CrossRef]
- Bordiga, M.; Rinaldi, M.; Locatelli, M.; Piana, G.; Travaglia, F.; Coisson, J.D.; Arlorio, M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013, 140, 57–67. [Google Scholar] [CrossRef]
- Stamatopoulos, P.; Frerot, E.; Tempere, S.; Pons, A.; Darriet, P. Identification of a New Lactone Contributing to Overripe Orange Aroma in Bordeaux Dessert Wines via Perceptual Interaction Phenomena. J. Agric. Food Chem. 2014, 62, 2469–2478. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Welke Juliane, E.; Zanus, M.; Lazzarotto, M.; Pulgati Fernando, H.; Zini Claudia, A. Main differences between volatiles of sparkling and base wines accessed through comprehensive two dimensional gas chromatography with time-of-flight mass spectrometric detection and chemometric tools. Food Chem 2014, 164, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, K.P.; Biasoto, A.C.T.; Souza-Silva, E.A.; Guerra, C.C.; dos Santos, H.P.; Welke, J.E.; Zini, C.A. Sensory, olfactometry and comprehensive two-dimensional gas chromatography analyses as appropriate tools to characterize the effects of vine management on wine aroma. Food Chem. 2018, 243, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Lusk, L.T.; Kay, S.B.; Porubcan, A.; Ryder, D.S. Key olfactory cues for beer oxidation. J. Amer. Soc. Brewing Chem. 2012, 70, 257–261. [Google Scholar] [CrossRef]
- Martins, C.; Brandao, T.; Almeida, A.; Rocha, S.M. Insights on beer volatile profile: Optimization of solid-phase microextraction procedure taking advantage of the comprehensive two-dimensional gas chromatography structured separation. J. Sep. Sci. 2015, 38, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.-H.; Perrault, K.A.; Dubois, L.M.; L’Homme, B.; Allen, C.; Loughnane, C.; Ochiai, N.; Focant, J.-F. Advanced method optimization for volatile aroma profiling of beer using two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chrom. A 2017, 1507, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Eyres, G.; Dufour, J.-P.; Marriott, P.J. The spicy character of hops. Brewing and Distilling: A Common Heritage. Presented at the IBD CD-ROM Proceedings, Hobart, Tasmania, Australia, 19–24 March 2006. [Google Scholar]
- Eyres, G.T.; Marriott, P.J.; Dufour, J.-P. Comparison of Odor-Active Compounds in the Spicy Fraction of Hop (Humulus lupulus L.) Essential Oil from Four Different Varieties. J. Agric. Food Chem. 2007, 55, 6252–6261. [Google Scholar] [CrossRef]
- Sanekata, A.; Tanigawa, A.; Takoi, K.; Nakayama, Y.; Tsuchiya, Y. Identification and Characterization of Geranic Acid as a Unique Flavor Compound of Hops (Humulus lupulus L.) Variety Sorachi Ace. J. Agric. Food Chem. 2018, 66, 12285–12295. [Google Scholar] [CrossRef] [PubMed]
- Villiere, A.; Arvisenet, G.; Lethuaut, L.; Prost, C.; Serot, T. Selection of a representative extraction method for the analysis of odourant volatile composition of French cider by GC-MS-O and GC × GC-TOF-MS. Food Chem. 2012, 131, 1561–1568. [Google Scholar] [CrossRef]
- Wisniewska, P.; Sliwinska, M.; Dymerski, T.; Wardencki, W.; Namiesnik, J. Qualitative characteristics and comparison of volatile fraction of vodkas made from different botanical materials by comprehensive two-dimensional gas chromatography and the electronic nose based on the technology of ultra-fast gas chromatography. J. Sci. Food Agric. 2017, 97, 1316–1325. [Google Scholar] [CrossRef]
- Cardeal, Z.L.; Marriott, P.J. Comprehensive two-dimensional gas chromatography-mass spectrometry analysis and comparison of volatile organic compounds in Brazilian cachaca and selected spirits. Food Chem. 2008, 112, 747–755. [Google Scholar] [CrossRef]
- Capobiango, M.; Mastello, R.B.; Chin, S.-T.; Oliveira, E.d.S.; Cardeal, Z.d.L.; Marriott, P.J. Identification of aroma-active volatiles in banana Terra spirit using multidimensional gas chromatography with simultaneous mass spectrometry and olfactometry detection. J. Chrom. A 2015, 1388, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Waseem, R.; Low, K.H. Advanced analytical techniques for the extraction and characterization of plant-derived essential oils by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 483–501. [Google Scholar] [CrossRef]
- Mondello, L.; Casilli, A.; Tranchida, P.Q.; Dugo, G.; Dugo, P. Comprehensive two-dimensional gas chromatography in combination with rapid scanning quadrupole mass spectrometry in perfume analysis. J. Chrom. A 2005, 1067, 235–243. [Google Scholar] [CrossRef]
- Doetterl, S.; Burkhardt, D.; Weissbecker, B.; Juergens, A.; Schuetz, S.; Mosandl, A. Linalool and lilac aldehyde/alcohol in flower scents. Electrophysiological detection of lilac aldehyde stereoisomers by moth. J. Chrom. A 2006, 1113, 231–238. [Google Scholar] [CrossRef]
- Phi, N.T.L.; Tu, N.T.M.; Nishiyama, C.; Sawamura, M. Characterisation of the odour volatiles in Citrus aurantifolia Persa lime oil from Vietnam. Develop. Food Sci. 2006, 43, 193–196. [Google Scholar]
- D’Acampora Zellner, B.; Casilli, A.; Dugo, P.; Dugo, G.; Mondello, L. Odor fingerprint acquisition by means of comprehensive two-dimensional gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chrom. A 2007, 1141, 279–286. [Google Scholar] [CrossRef]
- Breme, K.; Tournayre, P.; Fernandez, X.; Meierhenrich Uwe, J.; Brevard, H.; Joulain, D.; Berdague Jean, L. Identification of odor impact compounds of Tagetes minuta L. essential oil: Comparison of two GC-olfactometry methods. J Agric Food Chem 2009, 57, 8572–8580. [Google Scholar] [CrossRef]
- Fonseca de Godoy, L.A.; Hantao, L.W.; Pedroso, M.P.; Poppi, R.J.; Augusto, F. Quantitative analysis of essential oils in perfume using multivariate curve resolution combined with comprehensive two-dimensional gas chromatography. Analy. Chim. Acta 2011, 699, 120–125. [Google Scholar] [CrossRef]
- Fidelis, C.H.V.; Sampaio, P.T.B.; Krainovic, P.M.; Augusto, F.; Barata, L.E.S. Correlation between maturity of tree and GC × GC-qMS chemical profiles of essential oil from leaves of Aniba rosaeodora Ducke. Microchem. J. 2013, 109, 73–77. [Google Scholar] [CrossRef]
- Filippi, J.-J.; Belhassen, E.; Baldovini, N.; Brevard, H.; Meierhenrich, U.J. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chrom. A 2013, 1288, 127–148. [Google Scholar] [CrossRef]
- Van der Wat, L.; Dovey, M.; Naude, Y.; Forbes, P.B.C. Investigation into the aroma of rosemary using multi-channel silicone rubber traps, off-line olfactometry and comprehensive two-dimensional gas chromatography-mass spectrometry. South Afric. J. Chem. 2013, 66, 21–26. [Google Scholar]
- Tan, H.P.; Wan, T.S.; Min, C.L.S.; Osborne, M.; Ng, K.H. Quantitative analysis of fragrance in selectable one dimensional or two dimensional gas chromatography-mass spectrometry with simultaneous detection of multiple detectors in single injection. J. Chrom. A 2014, 1333, 106–115. [Google Scholar] [CrossRef]
- Notar Francesco, I.; Filippi, J.-J.; Antoniotti, S. Sustainable manufacture of a valuable fragrance ingredient: Lipase-catalyzed acylation of vetiver essential oil and chemoselectivity between sesquiterpene alcohols. ChemPlusChem 2017, 82, 407–415. [Google Scholar] [CrossRef]
- Tissandie, L.; Brevard, H.; Belhassen, E.; Alberola, M.; Meierhenrich, U.; Filippi, J.-J. Integrated comprehensive two-dimensional gas-chromatographic and spectroscopic characterization of vetiveryl acetates: Molecular identifications, quantification of constituents, regulatory and olfactory considerations. J. Chrom. A 2018, 1573, 125–150. [Google Scholar] [CrossRef]
- Zure, C.V.; Pinjari, R.V. Development and validation of 2D GC-FID method for quantitative analysis of cis- and trans-hexyl cinnamic aldehyde and its major impurity 2-hexyl-2-decenal. Asian J. Chem. 2018, 30, 1088–1092. [Google Scholar] [CrossRef]
- Dunn, M.S.; Vulic, N.; Shellie, R.A.; Whitehead, S.; Morrison, P.; Marriott, P.J. Targeted multidimensional gas chromatography for the quantitative analysis of suspected allergens in fragrance products. J. Chrom. A 2006, 1130, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Devos, C.; Ochiai, N.; Sasamoto, K.; Sandra, P.; David, F. Full evaporation dynamic headspace in combination with selectable one-dimensional/two-dimensional gas chromatography-mass spectrometry for the determination of suspected fragrance allergens in cosmetic products. J. Chrom. A 2012, 1255, 207–215. [Google Scholar] [CrossRef]
- Wong, Y.F.; Perlmutter, P.; Marriott, P.J. Untargeted metabolic profiling of Eucalyptus spp. leaf oils using comprehensive two-dimensional gas chromatography with high resolution mass spectrometry: Expanding the metabolic coverage. Metabolomics 2017, 13, 1–17. [Google Scholar] [CrossRef]
- Morrison, P.D.; Marriott, P.J.; Poynter, S.D.H.; Shellie, R.A. Selection of columns for GCxGC analysis of essential oils. LC-GC Europe 2010, 23, 78–80. [Google Scholar]
- Shellie, R.A.; Marriott, P.J. Comprehensive two-dimensional gas chromatography-mass spectrometry analysis of Pelargonium graveolens essential oil using rapid scanning quadrupole mass spectrometry. Analyst 2003, 128, 879–883. [Google Scholar] [CrossRef]
- Wong, Y.F.; Chin, S.-T.; Perlmutter, P.; Marriott, P.J. Evaluation of comprehensive two-dimensional gas chromatography with accurate mass time-of-flight mass spectrometry for the metabolic profiling of plant-fungus interaction in Aquilaria malaccensis. J. Chrom. A 2015, 1387, 104–115. [Google Scholar] [CrossRef]
- Wong, Y.F.; Davies, N.W.; Chin, S.-T.; Larkman, T.; Marriott, P.J. Enantiomeric distribution of selected terpenes for authenticity assessment of Australian Melaleuca alternifolia oil. Ind. Crops Prod. 2015, 67, 475–483. [Google Scholar] [CrossRef]
- Elbashir, A.A.; Aboul-Enein, H.Y. Multidimensional Gas Chromatography for Chiral Analysis. Crit. Rev. Analy. Chem. 2018, 48, 416–427. [Google Scholar] [CrossRef]
- Marriott, P.J.; Eyres, G.T.; Dufour, J.-P. Emerging Opportunities for Flavor Analysis through Hyphenated Gas Chromatography. J. Agric. Food Chem. 2009, 57, 9962–9971. [Google Scholar] [CrossRef] [PubMed]
- Marriott, P.J.; Chin, S.-T.; Maikhunthod, B.; Schmarr, H.-G.; Bieri, S. Multidimensional gas chromatography. TrAC Trends Analy. Chem. 2012, 34, 1–21. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Franchina, F.A.; Mondello, L. Analysis of essential oils through comprehensive two-dimensional gas chromatography: General utility. Flavour Fragr. J. 2017, 32, 218–227. [Google Scholar] [CrossRef]
- Lebanov, L.; Tedone, L.; Kaykhaii, M.; Linford, M.R.; Paull, B. Multidimensional Gas Chromatography in Essential Oil Analysis. Part 2: Application to Characterisation and Identification. Chromatographia 2018. In press. [Google Scholar] [CrossRef]
- Dunn, M.; Shellie, R.; Morrison, P.; Marriott, P. Rapid sequential heart-cut multidimensional gas chromatographic analysis. J. Chrom. A 2004, 1056, 163–169. [Google Scholar] [CrossRef]
- Schreiner, L.; Loos, H.M.; Buettner, A. Identification of odorants in wood of Calocedrus decurrens (Torr.) Florin by aroma extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry. Analy. BioAnaly. Chem. 2017, 409, 3719–3729. [Google Scholar] [CrossRef] [PubMed]
- Ghadiriasli, R.; Wagenstaller, M.; Buettner, A. Identification of odorous compounds in oak wood using odor extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry. Analy. BioAnaly. Chem. 2018, 410, 6595–6607. [Google Scholar] [CrossRef]
- Tissandie, L.; Viciana, S.; Brevard, H.; Meierhenrich, U.J.; Filippi, J.-J. Towards a complete characterization of guaiacwood oil. Phytochemistry 2018, 149, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the smell of wood-identification of odour-active compounds in Scots pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Slabizki, P.; Schmarr, H.-G. Analysis of corky off-flavour compounds at ultra trace level with multidimensional gas chromatography-electron capture detection. J. Chrom. A 2013, 1271, 181–184. [Google Scholar] [CrossRef]
- Slabizki, P.; Fischer, C.; Legrum, C.; Schmarr, H.-G. Characterization of Atypical Off-Flavor Compounds in Natural Cork Stoppers by Multidimensional Gas Chromatographic Techniques. J. Agric. Food Chem. 2015, 63, 7840–7848. [Google Scholar] [CrossRef]
- Tran, T.C.; Marriott, P.J. Characterization of incense smoke by solid phase microextraction-Comprehensive two-dimensional gas chromatography (GC × GC). Atmospheric Environ. 2007, 41, 5756–5768. [Google Scholar] [CrossRef]
- Tran, T.C.; Marriott, P.J. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and simultaneous electron capture detection/nitrogen phosphorous detection for incense analysis. Atmospheric Environ. 2008, 42, 7360–7372. [Google Scholar] [CrossRef]
- Jiang, M.; Kulsing, C.; Marriott, P.J. Comprehensive 2D gas chromatography-time-of-flight mass spectrometry with 2D retention indices for analysis of volatile compounds in frankincense (Boswellia papyrifera). Analy. BioAnaly. Chem. 2018, 410, 3185–3196. [Google Scholar] [CrossRef]
- Niebler, J.; Buettner, A. Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry. Phytochemistry 2015, 109, 66–75. [Google Scholar] [CrossRef]
- Gordon, B.M.; Uhrig, M.S.; Borgerding, M.F.; Chung, H.L.; Coleman, W.M., III; Elder, J.F., Jr.; Giles, J.A.; Moore, D.S.; Rix, C.E.; White, E.L. Analysis of flue-cured tobacco essential oil by hyphenated analytical techniques. J. Chromatogr. Sci. 1988, 26, 174–180. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Liu, S.; Yu, H.; Dai, Y. Analytical method of free and conjugated neutral aroma components in tobacco by solvent extraction coupled with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J. Chrom. A 2013, 1280, 122–127. [Google Scholar] [CrossRef]
- Wang, J.-A.; Yang, G.-H.; Li, C.-X. Zonal distribution of neutral aroma components in flue-cured tobacco leaves. Phytochem. Lett. 2018, 24, 125–130. [Google Scholar] [CrossRef]
- Ochiai, N.; Mitsui, K.; Sasamoto, K.; Yoshimura, Y.; David, F.; Sandra, P. Multidimensional gas chromatography in combination with accurate mass, tandem mass spectrometry, and element-specific detection for identification of sulfur compounds in tobacco smoke. J. Chrom. A 2014, 1358, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Xu, Z.; Shu, J.; Sun, W.; Yin, C.; Chen, M.; Li, Y.; Zhong, F. Quantitative determination of the major aroma compounds in cigarette smoke condensates using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry based on direct solvent extraction and comparison with simultaneous distillation extraction. Analy. Methods 2013, 5, 3557–3564. [Google Scholar]
- Bylinski, H.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Complementary use of GCxGC-TOF-MS and statistics for differentiation of variety in biosolid samples. Monatshefte fuer Chemie 2018, 149, 1587–1594. [Google Scholar] [CrossRef]
- Bylinski, H.; Kolasinska, P.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Determination of odour concentration by TD-GC×GC-TOF-MS and field olfactometry techniques. Monatshefte fuer Chemie 2017, 148, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Yu, J.; Zhang, H.; Zhang, Y.; Yang, M.; Lu, N.; Zhang, D. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry for the screening of potent swampy/septic odor-causing compounds in two drinking water sources in China. Analy. Methods 2015, 7, 2458–2468. [Google Scholar] [CrossRef]
- Guo, Q.; Yu, J.; Yang, K.; Wen, X.; Zhang, H.; Yu, Z.; Li, H.; Zhang, D.; Yang, M. Identification of complex septic odorants in Huangpu River source water by combining the data from gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography using retention indices. Sci. Total Environ. 2016, 556, 36–44. [Google Scholar] [CrossRef]
- Rong, C.; Liu, D.; Li, Y.; Yang, K.; Han, X.; Pan, B.; Zhang, J.; Rong, C.; Zhang, J.; Liu, D.; et al. Source water odor in one reservoir in hot and humid areas of southern China: Occurrence, diagnosis and possible mitigation measures. Environ. Sci. Euro. 2018, 30, 45. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Koziel, J.A.; O’Neal, M.E. Why do Ladybugs Smell Bad? In-vivo Quantification of Odorous Insect Kairomones with SPME and Multidimensional GC-MS-Olfactometry. AIP Conference Proc. 2009, 1137, 245–248. [Google Scholar] [Green Version]
- Saraiva, M.J.; Salvador, A.C.; Fernandes, T.; Ferreira, J.P.; Barros, A.S.; Rocha, S.M.; Fonseca, C. Three mammal species distinction through the analysis of scats chemical composition provided by comprehensive two-dimensional gas chromatography. Biochem. Systematics Ecol. 2014, 55, 46–52. [Google Scholar] [CrossRef]
- Ueland, M.; Ewart, K.; Troobnikoff, A.N.; Frankham, G.; Johnson, R.N.; Forbes, S.L. A rapid chemical odour profiling method for the identification of rhinoceros horns. Forensic Sci. Int. 2016, 266, e99–e102. [Google Scholar] [CrossRef] [PubMed]
- Buettner, A. A selective and sensitive approach to characterize odor-active and volatile constituents in small-scale human milk samples. Flavour Fragr. J. 2007, 22, 465–473. [Google Scholar] [CrossRef]
- Kuhn, F.; Natsch, A. Body odor of monozygotic human twins: A common pattern of odorant carboxylic acids released by a bacterial aminoacylase from axilla secretions contributing to an inherited body odour type. J. Royal Soc. Interface 2009, 6, 377–392. [Google Scholar] [CrossRef]
- Dolezal, P.; Kyjakova, P.; Valterova, I.; Urban, S. Qualitative analyses of less-volatile organic molecules from female skin scents by comprehensive two dimensional gas chromatography-time of flight mass spectrometry. J. Chrom. A 2017, 1505, 77–86. [Google Scholar] [CrossRef]
- De la Mata, A.P.; Nam Seo, L.; Harynuk James, J.; McQueen Rachel, H. Comprehensive two-dimensional gas chromatographic profiling and chemometric interpretation of the volatile profiles of sweat in knit fabrics. Anal. Bioanal. Chem. 2017, 409, 1905–1913. [Google Scholar] [CrossRef]
- Gruber, B.; Weggler, B.A.; Jaramillo, R.; Murrell, K.A.; Piotrowski, P.K.; Dorman, F.L. Comprehensive two-dimensional gas chromatography in forensic science: A critical review of recent trends. TrAC Trends Analy. Chem. 2018, 105, 292–301. [Google Scholar] [CrossRef]
- Rust, L.; Nizio, K.D.; Wand, M.P.; Forbes, S.L. Investigating the detection limits of scent-detection dogs to residual blood odour on clothing. Forensic Chem. 2018, 9, 62–75. [Google Scholar] [CrossRef]
- Cuzuel, V.; Leconte, R.; Cognon, G.; Thiebaut, D.; Vial, J.; Sauleau, C.; Rivals, I. Human odor and forensics: Towards Bayesian suspect identification using GC × GC-MS characterization of hand odor. J. Chromatogr. B Analy. Technol. Biomed. Life Sci. 2018, 1092, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Cuzuel, V.; Sizun, A.; Cognon, G.; Rivals, I.; Heulard, F.; Thiebaut, D.; Vial, J. Human odor and forensics. Optimization of a comprehensive two-dimensional gas chromatography method based on orthogonality: How not to choose between criteria. J. Chrom. A 2018, 1536, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chilcote, B.; Rust, L.; Nizio Katie, D.; Forbes Shari, L. Profiling the scent of weathered training aids for blood-detection dogs. Sci. Justice J. Forensic Sci. Soc. 2018, 58, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.M.; Perrault, K.A.; Stefanuto, P.-H.; Koschinski, S.; Edwards, M.; McGregor, L.; Focant, J.-F. Thermal desorption comprehensive two-dimensional gas chromatography coupled to variable-energy electron ionization time-of-flight mass spectrometry for monitoring subtle changes in volatile organic compound profiles of human blood. J. Chrom. A 2017, 1501, 117–127. [Google Scholar] [CrossRef]
- Stadler, S.; Stefanuto, P.-H.; Byer, J.D.; Brokl, M.; Forbes, S.; Focant, J.-F. Analysis of synthetic canine training aids by comprehensive two-dimensional gas chromatography-time of flight mass spectrometry. J. Chrom. A 2012, 1255, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.M.; Stefanuto, P.-H.; Heudt, L.; Focant, J.-F.; Perrault, K.A. Characterizing decomposition odor from soil and adipocere samples at a death scene using HS-SPME-GC×GC-HRTOFMS. Forensic Chem. 2018, 8, 11–20. [Google Scholar] [CrossRef]
- Stefanuto, P.H.; Perrault, K.A.; Lloyd, R.M.; Stuart, B.; Rai, T.; Forbes, S.L.; Focant, J.F. Exploring new dimensions in cadaveric decomposition odour analysis. Analy. Methods 2015, 7, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Stadler, S.; Stefanuto, P.-H.; Brokl, M.; Forbes, S.L.; Focant, J.-F. Characterization of Volatile Organic Compounds from Human Analogue Decomposition Using Thermal Desorption Coupled to Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry. Analy. Chem. 2013, 85, 998–1005. [Google Scholar] [CrossRef]
- Brasseur, C.; Dekeirsschieter, J.; Schotsmans, E.M.J.; de Koning, S.; Wilson, A.S.; Haubruge, E.; Focant, J.-F. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J. Chrom. A 2012, 1255, 163–170. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Stefanuto, P.-H.; Brasseur, C.; Haubruge, E.; Focant, J.-F. Enhanced characterization of the smell of death by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOFMS). PLoS ONE 2012, 7, e39005. [Google Scholar] [CrossRef]
- Forbes, S.L.; Perrault, K.A.; Stefanuto, P.-H.; Nizio, K.D.; Focant, J.-F. Comparison of the decomposition VOC profile during winter and summer in a moist, mid-latitude (Cfb) climate. PLoS ONE 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Perrault, K.A.; Stefanuto, P.-H.; Stuart, B.H.; Rai, T.; Focant, J.-F.; Forbes, S.L. Detection of decomposition volatile organic compounds in soil following removal of remains from a surface deposition site. Forensic Sci. Med. Pathol. 2015, 11, 376–387. [Google Scholar] [CrossRef]
- Perrault, K.A.; Stefanuto, P.-H.; Stuart, B.H.; Rai, T.; Focant, J.-F.; Forbes, S.L. Reducing variation in decomposition odor profiling using comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2015, 38, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.-H.; Perrault, K.; Stadler, S.; Pesesse, R.; Brokl, M.; Forbes, S.; Focant, J.-F. Reading Cadaveric Decomposition Chemistry with a New Pair of Glasses. ChemPlusChem 2014, 79, 786–789. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Grabherr, S.; Varlet, V.; Focant, J.-F. Postmortem internal gas reservoir monitoring using GC×GC-HRTOF-MS. Separations 2016, 3, 24. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Stadler, S.; Pesesse, R.; LeBlanc, H.N.; Forbes, S.L.; Focant, J.-F. GC × GC-TOFMS and supervised multivariate approaches to study human cadaveric decomposition olfactive signatures. Analy. BioAnaly. Chem. 2015, 407, 4767–4778. [Google Scholar] [CrossRef]
- De Vos, J.; Dixon, R.; Vermeulen, G.; Gorst-Allman, P.; Cochran, J.; Rohwer, E.; Focant, J.-F. Comprehensive two-dimensional gas chromatography time of flight mass spectrometry (GC × GC-TOFMS) for environmental forensic investigations in developing countries. Chemosphere 2011, 82, 1230–1239. [Google Scholar] [CrossRef]
- Megson, D.; Reiner, E.J.; Jobst, K.J.; Dorman, F.L.; Robson, M.; Focant, J.-F. A review of the determination of persistent organic pollutants for environmental forensics investigations. Analy. Chim. Acta 2016, 941, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.-H.; Perrault, K.A.; Focant, J.-F.; Forbes, S.L. Fast chromatographic method for explosive profiling. Chromatography 2015, 2, 213–224. [Google Scholar] [CrossRef]
- Mitrevski, B.; Veleska, B.; Engel, E.; Wynne, P.; Song Shin, M.; Marriott Philip, J. Chemical signature of ecstasy volatiles by comprehensive two-dimensional gas chromatography. Forensic Sci. Int. 2011, 209, 11–20. [Google Scholar] [CrossRef]
- Mitrevski, B.; Wynne, P.; Marriott, P.J. Comprehensive two-dimensional gas chromatography applied to illicit drug analysis. Analy. BioAnaly. Chem. 2011, 401, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Koziel, J.A. The relationship between chemical concentration and odor activity value explains the inconsistency in making a comprehensive surrogate scent training tool representative of illicit drugs. Forensic Sci. Int. 2015, 257, 257–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, P.; Buettner, A.; Bauer, P.; Buettner, A. Characterization of Odorous and Potentially Harmful Substances in Artists’ Acrylic Paint. Frontiers Public Health 2018, 6, 350. [Google Scholar] [CrossRef]
- Denk, P.; Buettner, A. Identification and quantification of glue-like off-odors in elastic therapeutic tapes. Analy. BioAnaly. Chem. 2018, 410, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, C.; Buettner, A.; Wiedmer, C.; Buettner, A.; Velasco-Schon, C. Characterization of off-odours and potentially harmful substances in a fancy dress accessory handbag for children. Sci. Rep. 2017, 7, 1807. [Google Scholar] [CrossRef] [Green Version]
- Wiedmer, C.; Velasco-Schoen, C.; Buettner, A. Characterization of odorants in inflatable aquatic toys and swimming learning devices-which substances are causative for the characteristic odor and potentially harmful? Analy. BioAnaly. Chem. 2017, 409, 3905–3916. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. Scientists seek to sniff out diseases electronic “noses” may someday be diagnostic tools. JAMA J. Amer. Med. Assoc. 2009, 301, 585–586. [Google Scholar] [CrossRef]
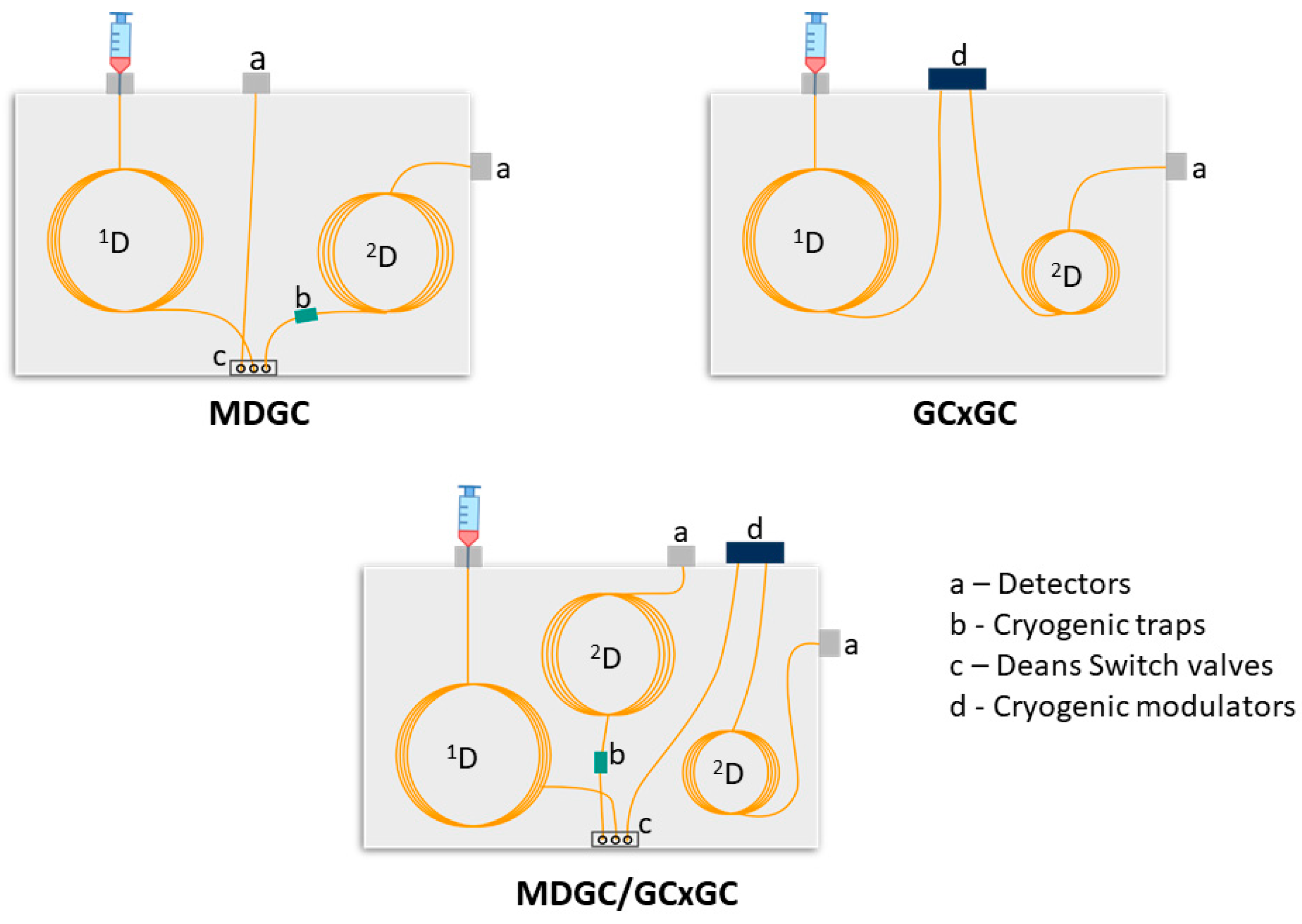
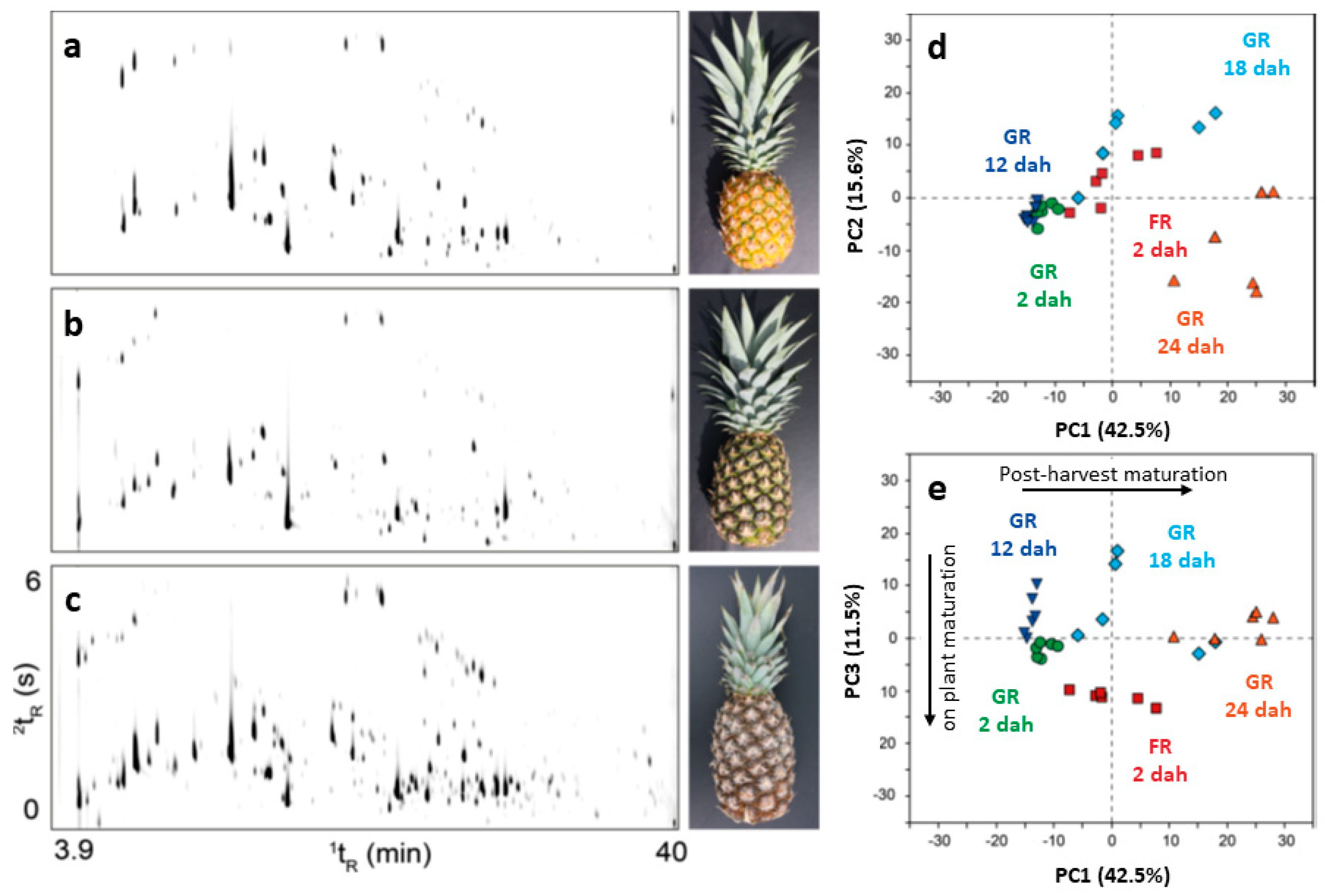


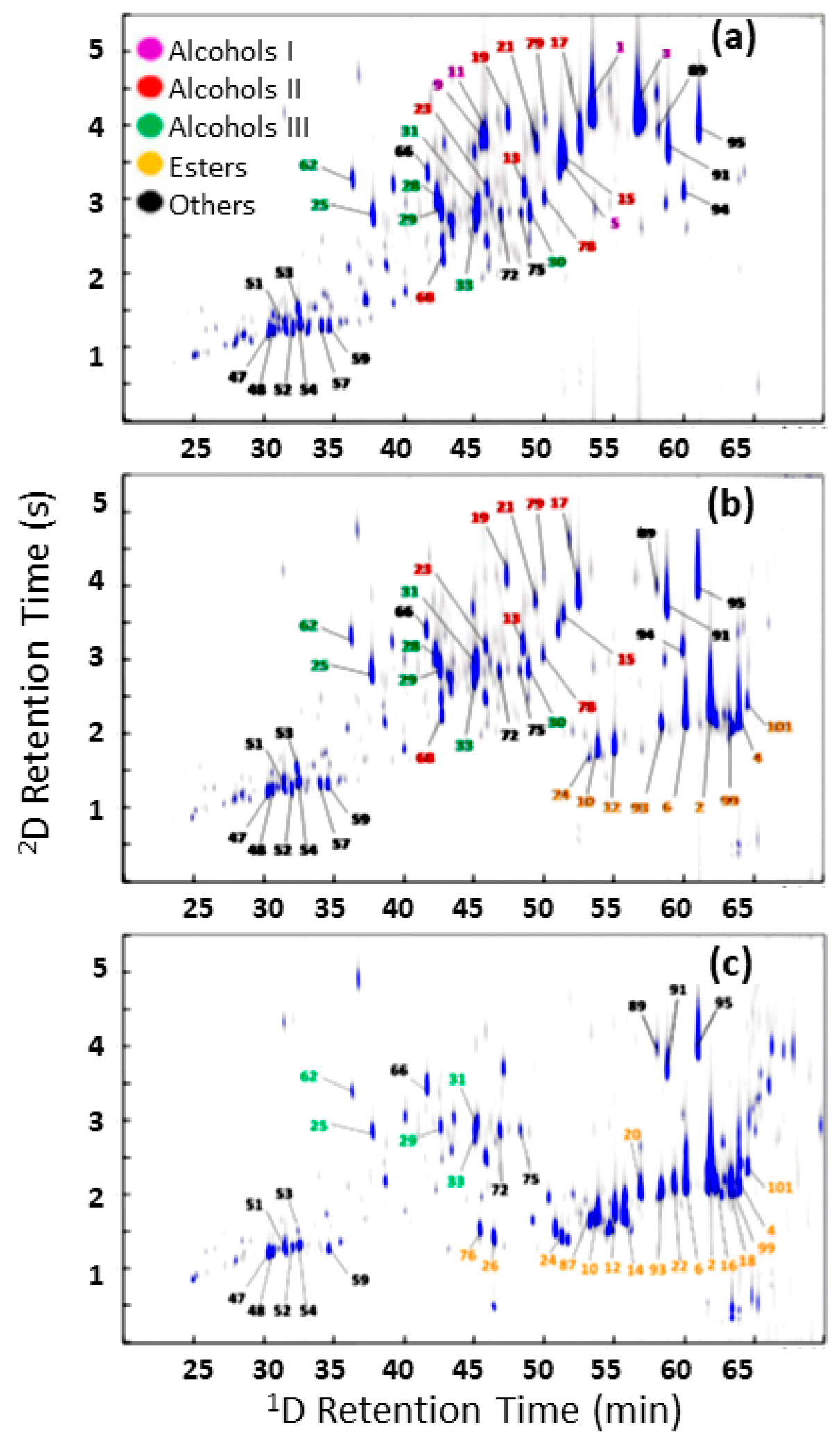
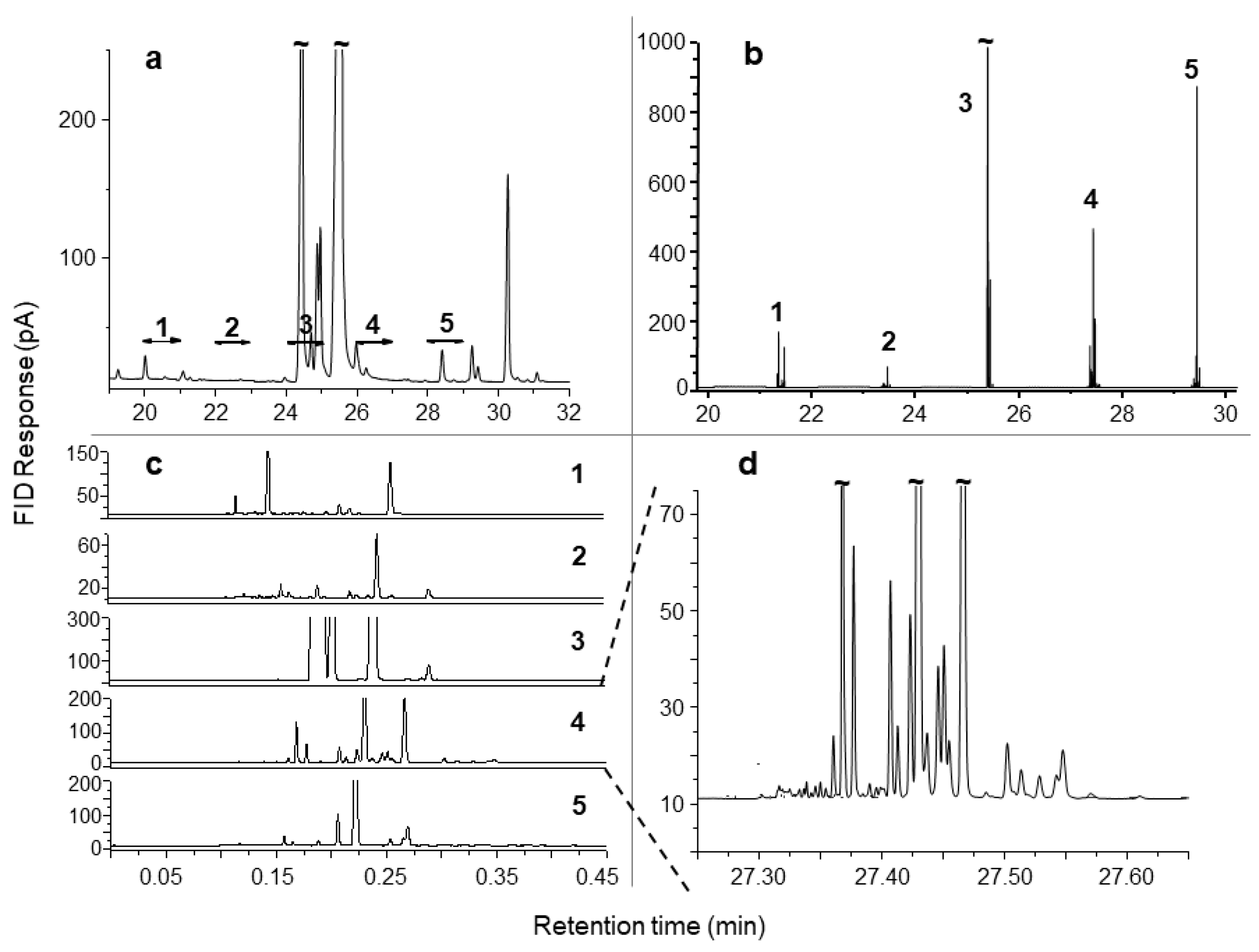
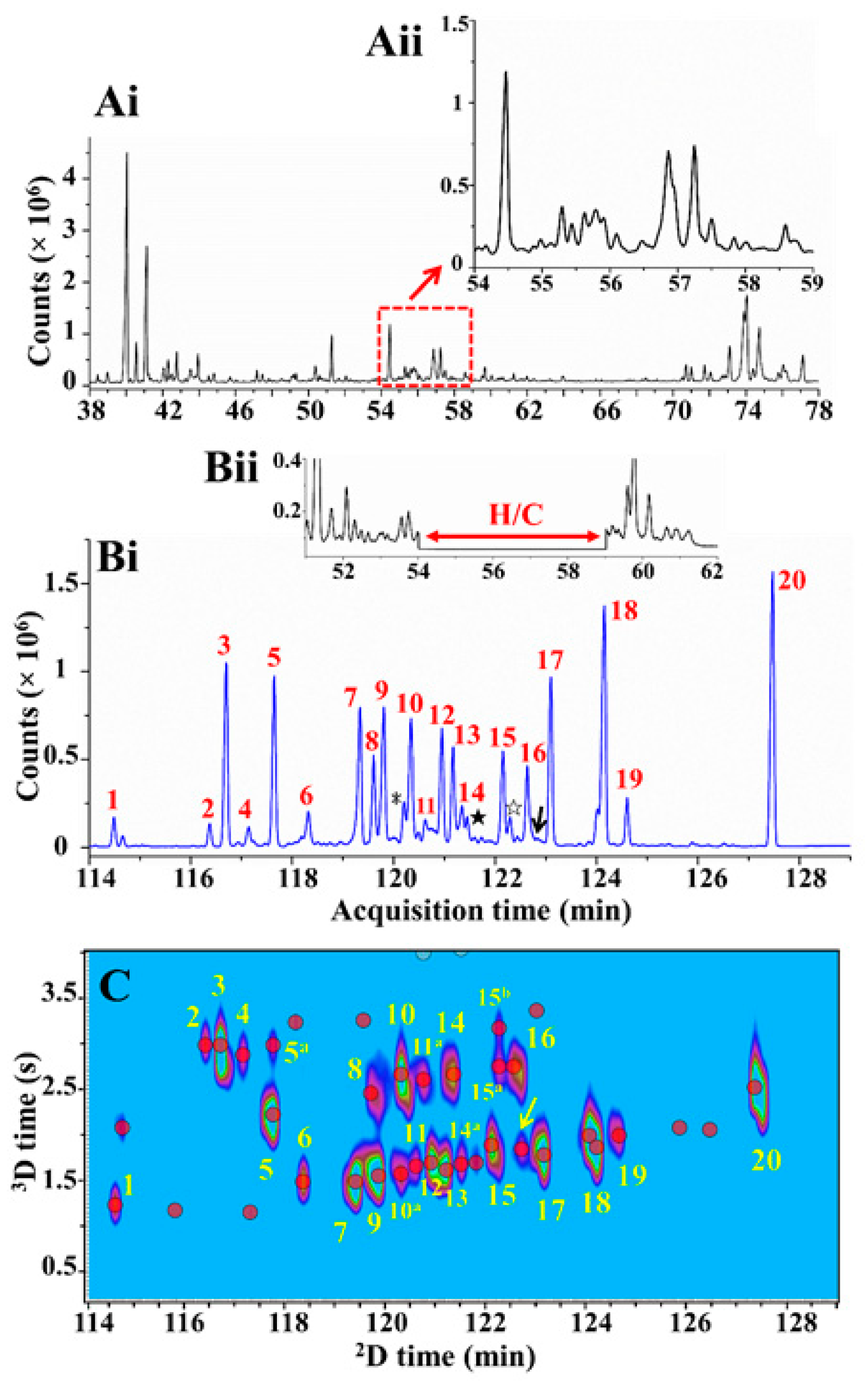
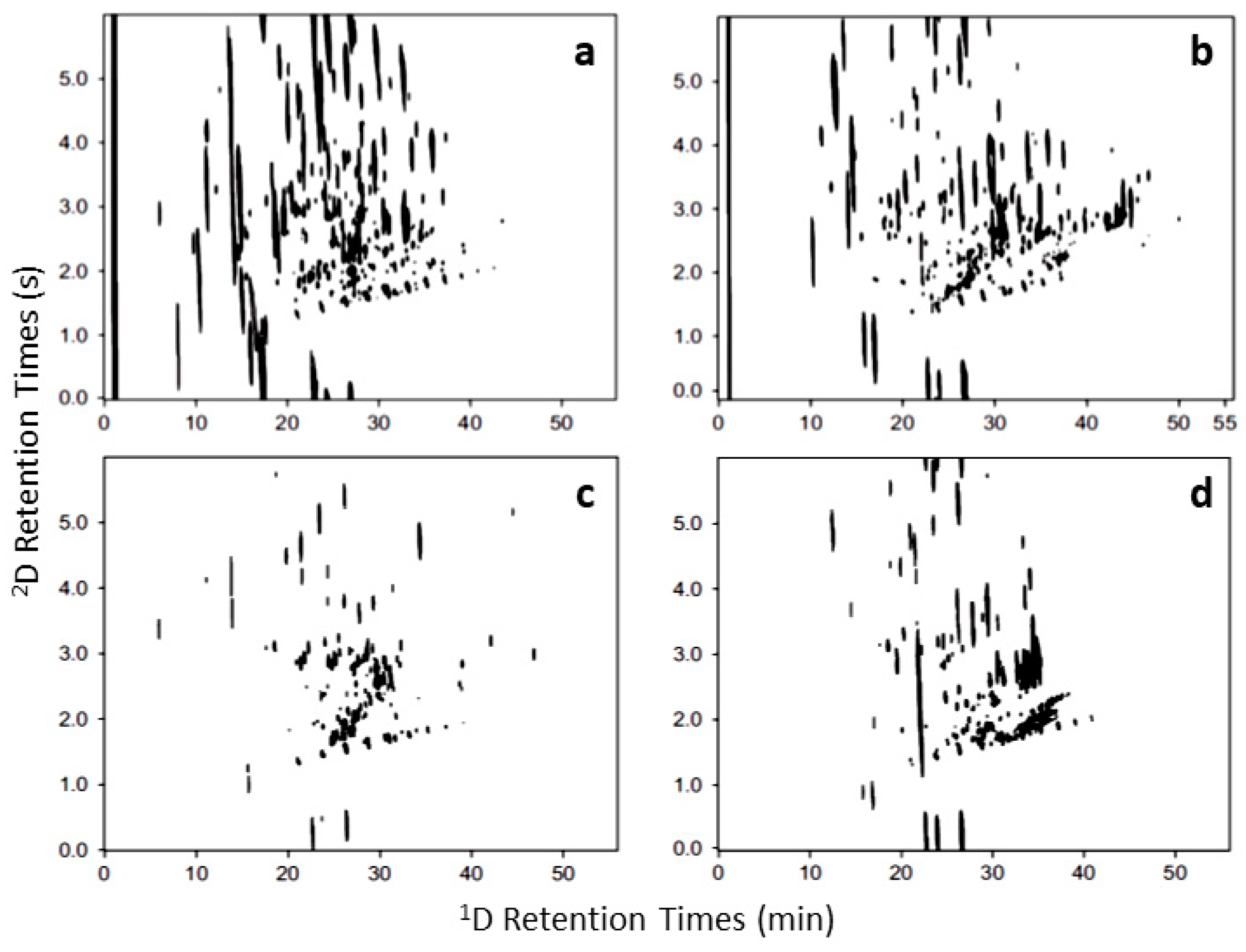

| Technique | Features | Pros | Cons |
|---|---|---|---|
| MDGC | - Target analysis (heart-cut; H/C) - Target peaks/regions are transferred according to retention times in 1D, and analysed in 2D - 2D column usually has similar dimensions to the 1D - A cryotrap can be used to focus the transferred analyte peaks/regions | - Allows enrichment of target analytes or fractions through multiple injections - Provides increased resolution on the 2D column and can improve detection specificity and sensitivity - Stereoisomer resolution uses a chiral 2D column and improves resolution from interfering peaks | - Requires a cryogenic trap to reduce 1D dispersion - Requires a switching valve for on-line H/C programming - Not designed to resolve the full sample; - Requires extra GC program to elute the 2D column, unless on-the-fly operation is used |
| GC×GC | - Non-target analysis; applied to all sample compounds - Modulator sub-samples peaks as small slices to 2D, giving a 2D plot - 2D ideally separates overlapped 1D peaks - 2D column is shorter, to separate transferred compounds before the next modulation | - Full 2D sample resolution can be achieved - Stereoisomer resolution uses a 1D chiral column - The 2D image generated provides excellent profiling/differentiation of samples. - Cryogenic modulation leads to response increase | - Requires a modulator; some can be costly - Method set-up can be more complex - Software and interpretation can be more convoluted - Personnel need special training |
| Samples/Column Sets * | References |
|---|---|
| Food | |
| Fruits and nuts: orange juice; Citrus spp.; pineapple; passion fruit; strawberry; raspberry; blue honeysuckle; chokeberry; bilberry; cocoa; hazelnuts. | [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] |
| Column sets: HP-FFAP/DB-5; Rtx-5MS/beta-DEXTM 55; Equity-1/SolGel-Wax | |
| Honey | |
| Column set: ZB5-MS/BPX50 | [38] |
| Dairy products: butter; dairy cream | |
| Columns sets: BP21/BPX35 | [39,40] |
| Flour and pasta | |
| Columns sets: SLB-5MS/Mega-Dex Det | [41] |
| Meats and Seafood: beef; lamb; ham; shrimp; seabass; eel | |
| Columns sets: DB-1/DB-225; HP-5 MS/BPX50; Rtx-5MS/DB-17 | [42,43,44,45,46,47,48,49] |
| Seasonings and related samples: coriander; curry leaves; fennel seeds; ginger; virgin olive oil; rapeseed oil; vinegar; tomato-onion puree; truffles. | |
| Columns sets: BPX5/BP20; HP-InnoWax/DB-1; Rxi-5Sil MS/BPX50; DB-WAX/DB-5 | [50,51,52,53,54,55,56,57,58,59,60,61,62] |
| Drinks and Beverages | |
| Non-alcoholic drinks: teas; coffee | [9,63,64,65,66,67,68,69,70,71,72,73] |
| Columns sets: Rxi-5Sil MS/Rxi-17; DB-5/Supelcowax; DB-Wax/Cyclosil B; TC-WAX/BETA DEX 225; DB-FFAP/DB-5 | |
| Alcoholic beverages: wine; brandy; beer and hop; cider; vodka; whiskey; cachaça; spirits | |
| Columns sets: DB-FFAP/DB-5; DB-5/DB-225; DB-WAX/DB-5; DB-1/DB-WAX | [8,9,14,15,68,69,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89] |
| Essential oils (E.O.) and fragrances | |
| flower odorants; vetiver E.O. and vetiveryl acetates (chemical and enzymatic products); rosewood leaves E.O.; eucalyptus E.O.; lavender E.O.; tea tree E.O.; peppermint E.O.; perfumes; allergens | [18,20,25,52,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116] |
| Columns sets: VF-5MS/DB-wax; DB-WAX/DB-5; Supelcowax/IL-59/BPX5 | |
| Wood | |
| guaiacwood; agarwood; oak wood; scots pine; cedar; cork | [12,109,117,118,119,120,121,122] |
| Columns sets: Supelcowax/Rxi-5Sil MS; VF-5MS/DB-wax; DB-FFAP/DB-5 | |
| Incenses | |
| lotus-scented; red Tibetan; medicine herb; brown (smokeless); frankincense | [123,124,125,126] |
| Columns sets: BPX5/BP20; DB-FFAP/Rxi-5 | |
| Tobacco and Cigarette | |
| tobacco leaves, tobacco smoke, cigarette smoke | [127,128,129,130,131] |
| Columns set: HP-5MS/DB-17 HT; DB-Wax/DB-1701; Rxi-5MS/Rxi-17 | |
| Environmental | |
| biosolid cakes; atmospheric air; water | [132,133,134,135,136] |
| Columns sets: Equity 1/SolGel-Wax; Rxi-5Silv/Rxi-17 | |
| Biologics | |
| ladybugs; mammal scats; rhinoceros horns; human milk; human sweat/skin odour | [137,138,139,140,141,142,143] |
| Columns sets: Rtx-5MS/DB-Wax; Rxi-624sil MS/Stabilwax | |
| Forensics | |
| pig and human cadavers; human remains; human hands scent; blood; environmental pollutants; | [144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167] |
| explosives; dog training mimetic odours; illicit drugs | |
| Columns sets: Rxi-624Sil MS/Stabilwax; Rxi-5Sil MS/Rxi-17Sil MS | |
| Plastic products | |
| children toys; balloons; balls; hairbrushes; inflatable pillows; elastic therapeutic tapes; acrylic paint | [168,169,170,171] |
| Columns set: DB-FFAP/DB-5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, M.S.S.; Marriott, P.J. The Blossoming of Technology for the Analysis of Complex Aroma Bouquets—A Review on Flavour and Odorant Multidimensional and Comprehensive Gas Chromatography Applications. Molecules 2019, 24, 2080. https://doi.org/10.3390/molecules24112080
Amaral MSS, Marriott PJ. The Blossoming of Technology for the Analysis of Complex Aroma Bouquets—A Review on Flavour and Odorant Multidimensional and Comprehensive Gas Chromatography Applications. Molecules. 2019; 24(11):2080. https://doi.org/10.3390/molecules24112080
Chicago/Turabian StyleAmaral, Michelle S.S., and Philip J. Marriott. 2019. "The Blossoming of Technology for the Analysis of Complex Aroma Bouquets—A Review on Flavour and Odorant Multidimensional and Comprehensive Gas Chromatography Applications" Molecules 24, no. 11: 2080. https://doi.org/10.3390/molecules24112080