An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production
Abstract
:1. Introduction
2. Results and Discussion
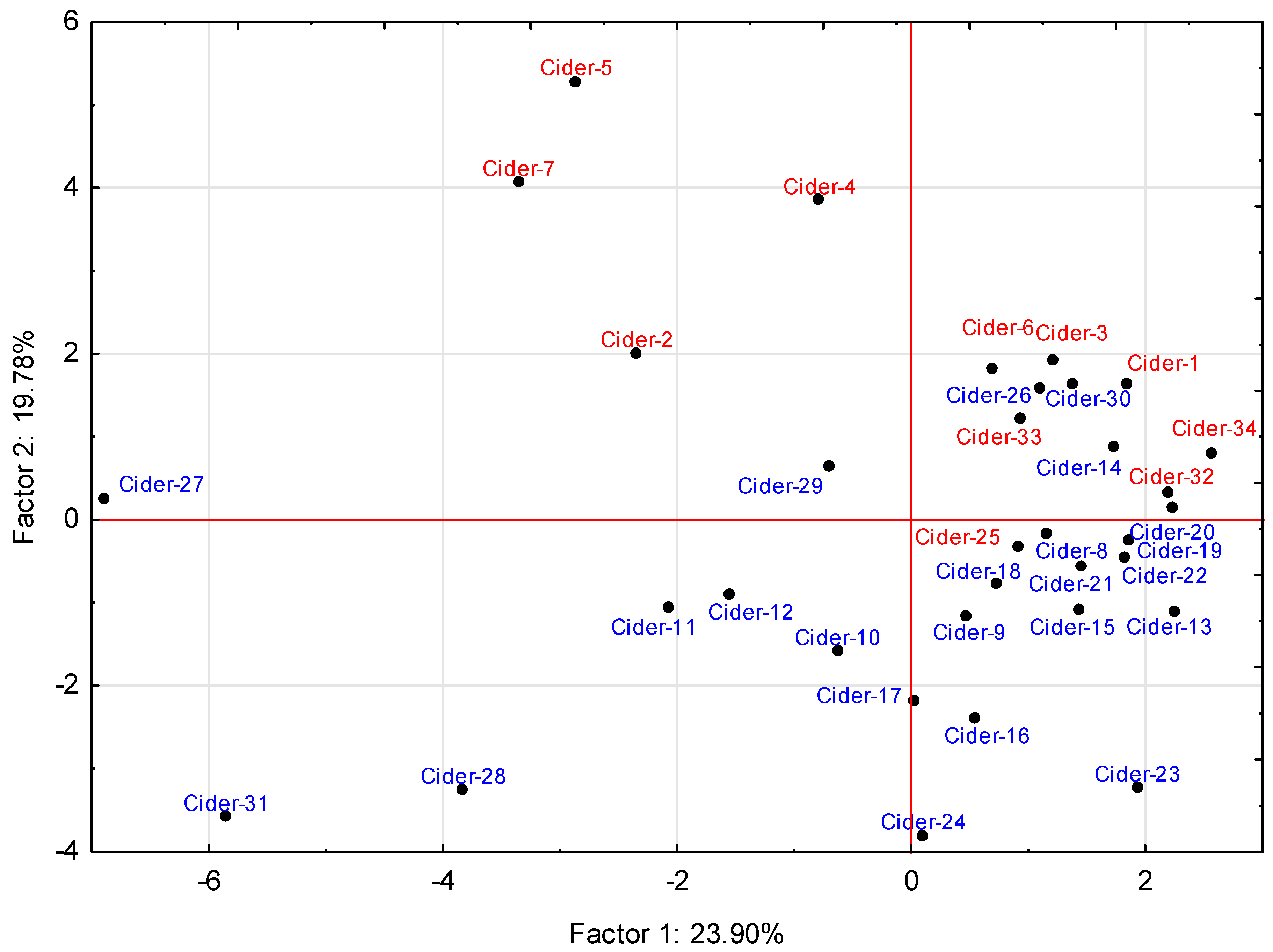
2.1. Variability of Volatiles in the Cider
2.2. Influence of Production Technology
2.3. Influence of Pitching Microorganism
3. Material and Method
3.1. Description of Samples
3.2. Reagents
3.3. Ethanol Content Determination
3.4. Sample Preparation
3.5. GC-MS Condition
3.6. Identification and Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- French:, R.K. The History and Virtues of Cyder; Robert Hale Limited: London, UK, 2010; p. 200. [Google Scholar]
- Anonymous. European Cider Trends 2018; European Cider and Fruit Wine Association: Brussels, Belgium, 2018; p. 24. Available online: http://aicv.org/file.handler?f=CiderTrends2018.pdf (accessed on 3 June 2019).
- Rye, G.G.; Mercer, D.G. Changes in headspace volatile attributes of apple cider resulting from thermal processing and storage. Food Res. Int. 2003, 36, 167–174. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.; Santos, L.H. Method optimization by solid-phase microextraction in combination with gas chromatography with mass spectrometry for analysis of beer volatile fraction. J. Chromatogr. A 2006, 1121, 145–153. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Noemí Palacios, G.; Ana García, H.; Belén Suárez, V. Application of purge and trap extraction and gas chromatography for determination of minor esters in cider. J. Chromatogr. A 2005, 1069, 245–251. [Google Scholar] [Green Version]
- Beech, F.W. Cider making and cider research—review. J. Inst. Brew. 1972, 78, 477–491. [Google Scholar] [CrossRef]
- Lea, A.G.H. Cidermaking. Fruit Process. 1995, 5, 281–286. [Google Scholar]
- Hui, Y.H.; Evranuz, E.Ö. Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; CRC Press: London, UK, Ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2012; p. 821. [Google Scholar]
- Xu, Y.; Fan, W.L.; Qian, M.C. Characterization of aroma compounds in apple cider using solvent-assisted flavor evaporation and headspace solid-phase microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef]
- Villière, A.; Arvisenet, G.; Lethuaut, L.; Prost, C.; Sérot, T. Selection of a representative extraction method for the analysis of odourant volatile composition of French cider by GC–MS–O and GC × GC–TOF-MS. Food Chem. 2012, 131, 1561–1568. [Google Scholar] [CrossRef]
- Fan, W.L.; Xu, Y.; Han, Y.H. Quantification of volatile compounds in Chinese ciders by stir bar sorptive extraction (SBSE) and gas chromatography-mass spectrometry (GC-MS). J. Inst. Brew. 2011, 117, 61–66. [Google Scholar] [CrossRef]
- Buglass, A.J. Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects; John Wiley & Sons: London, UK, 2011; p. 1208. [Google Scholar]
- Zhao, H.F.; Zhou, F.; Dziugan, P.; Ya, Y.H.; Zhang, J.T.; Lv, Z.L.; Zhang, B.L. Development of organic acids and volatile compounds in cider during malo lactic fermentation. Czech. J. Food Sci. 2014, 32, 69–76. [Google Scholar] [CrossRef]
- Williams, A.A.; Lewis, M.J.; Tucknott, O.G. The neutral volatile components of cider apple juices. Food Chem. 1980, 6, 139–151. [Google Scholar] [CrossRef]
- Verdu, C.F.; Gatto, J.; Freuze, I.; Richomme, P.; Laurens, F.; Guilet, D. Comparison of two methods, UHPLC-UV and UHPLC-MS/MS, for the quantification of polyphenols in cider apple juices. Molecules 2013, 18, 10213–10227. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. Lwt-Food Sci. Technol. 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Jarvis, B. Cider (cyder; hard cider) the product and its manufacture. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1312–1318. [Google Scholar]
- Food and Tobacco Products and on Amendments and Supplements to Certain Related Acts, for Non-Alcoholic Beverages and Concentrates for the Preparation of Soft Drinks, Fruit Wines, Other Wines and Mead, Beer, Alcohol, Spirits and Other Alcoholic Beverages, (A), (d), (h), (i), (j) and (k). Collection of Czech laws of 1997. Czech Law No. 335, No. 38. 1997.
- De la Commission du 14 juillet 2009 fixant certaines modalités d’application du règlement (ce) n° 479/2008 du conseil en ce qui concerne les appellations d’origine protégées et les indications géographiques protégées, les mentions traditionnelles, l’étiquetage et la présentation de certains produits du secteur vitivinicole. French Règlement (CE) n° 607. 2009.
- Qin, Z.H.; Petersen, M.A.; Bredie, W.L.P. Flavor profiling of apple ciders from the Uk and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef]
- Mangas, J.J.; González, M.P.; Rodríguez, R.; Blanco, D. Solid-phase extraction and determination of trace aroma and flavour components in cider by GC-MS. Chromatographia 1996, 42, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Garcia, L.A.; Diaz, M. Volatile compounds in cider: Inoculation time and fermentation temperature effects. J. Inst. Brew. 2006, 112, 210–214. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J. Synthesis of higher alcohols during cider processing. Food Chem. 1999, 67, 287–294. [Google Scholar] [CrossRef]
- Valappil, Z.A.; Fan, X.T.; Zhang, H.Q.; Rouseff, R.L. Impact of thermal and nonthermal processing technologies on unfermented apple cider aroma volatiles. J. Agric. Food Chem. 2009, 57, 924–929. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. 125th anniversary review: Diacetyl and its control during brewery fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar]
- Lin, J.; Jia, B.; Shan, S.S.; Xu, S.A. Fed-batch fermentation with glucose syrup as an adjunct for high-ethanol beer brewing. J. Inst. Brew. 2014, 120, 426–432. [Google Scholar] [CrossRef]
- Suárez Valles, B.; Pando Bedriñana, R.; Fernández Tascón, N.; Garcia Hevia, A.; Rodríguez Madrera, R. Analytical differentiation of cider inoculated with yeast (Saccharomyces cerevisiae) isolated from Asturian (Spain) apple juice. Lwt-Food Sci. Technol. 2005, 38, 455–461. [Google Scholar] [CrossRef]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- McKay, M.; Buglass, A.J.; Lee, C.G. Fermented beverages: Beers, ciders, wines and related drinks. In Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects; Buglass, A.J., Ed.; John Wiley & Sons: London, UK, 2011; pp. 63–454. [Google Scholar]
- Beech, F.W. 5-yeasts in cider-making. In The Yeasts, 2nd ed.; Harrison, A.H.R.S., Ed.; Academic Press: San Diego, CA, USA, 1993; pp. 169–213. [Google Scholar]
- Villiere, A.; Arvisenet, G.; Bauduin, R.; Le Quere, J.M.; Serot, T. Influence of cider-making process parameters on the odourant volatile composition of hard ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
- Madrera, R.R.; Bedrinana, R.P.; Valles, B.S. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT-Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Kelkar, S.; Dolan, K. Modeling the effects of initial nitrogen content and temperature on fermentation kinetics of hard cider. J. Food Eng. 2012, 109, 588–596. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.J.; Yin, H.; Chen, P.; Lin, H.; Chen, L. Monitoring of the production of flavour compounds by analysis of the gene transcription involved in higher alcohol and ester formation by the brewer’s yeast Saccharomyces pastorianus using a multiplex RT-QPCR assay. J. Inst. Brew. 2014, 120, 119–126. [Google Scholar] [CrossRef]
- Bleoanca, I.; Bahrim, G. Overview on brewing yeast stress factors. Rom. Biotechnol. Lett. 2013, 18, 8559–8572. [Google Scholar]
- Kruger, L.; Pickerell, A.T.W.; Axcell, B. The sensitivity of different brewing yeast strains to carbon-dioxide inhibition—fermentation and production of flavor-active volatile compounds. J. Inst. Brew. 1992, 98, 133–138. [Google Scholar] [CrossRef]
- Ciprian, T.; Rădulescu, A.; Codreşi, C.C.; Militaru, I.; Ovidiu, T. Aspects of the influence of filtration on qualitative and of composition of white wines. Bull. UASVM Hortic. 2011, 68, 1. [Google Scholar]
- Crook, L.R.; Boylston, T.D. Flavor characteristics of irradiated apple cider during storage: Effect of packaging materials and sorbate additon. J. Food Sci. 2004, 69, C557–C563. [Google Scholar] [CrossRef]
- Sun, S.Y.; Che, C.Y.; Sun, T.F.; Lv, Z.Z.; He, S.X.; Gu, H.N.; Shen, W.J.; Chi, D.C.; Gao, Y. Evaluation of sequential inoculation of saccharomyces cerevisiae and Oenococcus oeni strains on the chemical and aromatic profiles of cherry wines. Food Chem. 2013, 138, 2233–2241. [Google Scholar] [CrossRef]
- Ye, M.Q.; Yue, T.L.; Yuan, Y.H. Changes in the profile of volatile compounds and amino acids during cider fermentation using dessert variety of apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Strejc, J.; Siristova, L.; Karabin, M.; Silva, J.B.A.E.; Branyik, T. Production of alcohol-free beer with elevated amounts of flavouring compounds using lager yeast mutants. J. Inst. Brew. 2013, 119, 149–155. [Google Scholar] [CrossRef]
- Nespor, J.; Karabin, M.; Hanko, V.; Dostalek, P. Application of response surface design to optimise the chromatographic analysis of volatile compounds in beer. J. Inst. Brew. 2018, 124, 244–253. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |

| Sample Code | Concentration of Volatiles (mg/L) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethyl Acetate | Ethyl Butyrate | Ethyl 2-methyl Butyrate | Butyl Acetate | 2-Methyl Propanol | Isoamyl Acetate | 2-Methyl Butanol | 3-Methyl Butanol | Ethyl Hexanoate | Hexyl Acetate | Hexanol | Ethyl Octanoate | Acetic Acid | Butane-2,3-diol | Ethyl Decanoate | Diethyl Succinate | 2-Phenyl Ethyl Acetate | Hexanoic Acid | 2-Phenyl Ethanol | Ethyl tetradecanoate | Octanoic Acid | Decanoic Acid | |
| Cider-1 | 0.878 | 0.003 | 0.076 | 0.019 | 1.576 | 0.248 | 1.887 | 2.914 | 0.148 | 0.390 | 2.302 | 0.013 | 1.086 | 12.123 | 0.004 | 0.033 | 0.007 | 0.165 | 3.060 | 0.001 | 0.259 | 0.144 |
| Cider-2 | 6.792 | 0.004 | 0.147 | 0.004 | 0.803 | 2.626 | 0.755 | 0.376 | 0.743 | 0.378 | 0.703 | 0.195 | 3.697 | 3.152 | 0.016 | 0.131 | 0.036 | 2.410 | 0.960 | 0.002 | 3.348 | 1.530 |
| Cider-3 | 4.504 | 0.001 | 0.139 | 0.002 | 1.500 | 1.746 | 1.554 | 1.064 | 0.054 | 0.289 | 0.638 | 0.010 | 3.005 | 4.522 | 0.015 | 0.254 | n. d.* | 0.142 | 0.396 | 0.003 | 0.073 | 0.025 |
| Cider-4 | 6.862 | 0.022 | 0.366 | 0.397 | 1.053 | 1.237 | 2.950 | 1.045 | 1.063 | 1.473 | 1.907 | 0.020 | 0.000 | 10.847 | 0.011 | n. d.* | 0.065 | 0.415 | 1.222 | n. d.* | 0.518 | 0.003 |
| Cider-5 | 15.194 | 0.033 | 0.509 | 0.649 | 1.090 | 2.181 | 1.679 | 1.793 | 1.238 | 2.713 | 2.010 | 0.020 | 4.638 | 20.773 | 0.002 | n. d.* | 0.142 | 0.963 | 1.876 | n. d.* | 1.192 | 0.000 |
| Cider-6 | 8.390 | 0.009 | 0.042 | 0.044 | 0.872 | 0.367 | 1.576 | 0.063 | 1.139 | 0.119 | 0.416 | 0.014 | 2.654 | 2.784 | 0.021 | n. d.* | 0.094 | 0.854 | 1.246 | n. d.* | 0.904 | 0.044 |
| Cider-7 | 5.178 | 0.067 | 0.155 | 0.236 | 0.896 | 0.601 | 0.737 | 0.162 | 2.611 | 0.040 | 0.084 | 0.219 | 2.133 | n. d.* | 0.020 | n. d.* | 0.025 | 1.607 | 0.830 | 0.001 | 2.801 | 1.811 |
| Cider-8 | 20.150 | 0.002 | 0.006 | n. d.* | 1.079 | 0.753 | 2.114 | 1.589 | 0.379 | 0.008 | 0.902 | 0.097 | 13.280 | 6.656 | 0.015 | 0.518 | 0.010 | 0.751 | 1.968 | n. d.* | 1.491 | 0.786 |
| Cider-9 | 12.213 | 0.008 | 0.042 | 0.107 | 4.677 | 0.442 | 7.308 | 4.003 | 0.778 | 0.086 | 2.567 | 0.059 | 9.207 | 2.279 | 0.015 | 0.387 | 0.006 | 0.953 | 4.551 | n. d.* | 1.248 | 0.425 |
| Cider-10 | 8.710 | 0.002 | 0.003 | 0.001 | 3.351 | 0.125 | 4.420 | 5.816 | 0.414 | 0.029 | 3.541 | 0.055 | 4.824 | 6.112 | 0.051 | 0.048 | 0.067 | 4.880 | 14.786 | 0.001 | 0.713 | 1.313 |
| Cider-11 | 8.302 | 0.004 | 0.005 | 0.297 | 3.999 | 1.869 | 8.058 | 6.426 | 0.688 | 0.644 | 1.984 | 0.110 | 9.863 | 6.275 | 0.013 | 0.163 | 0.109 | 1.210 | 10.458 | n. d.* | 1.703 | 0.829 |
| Cider-12 | 9.646 | 0.005 | 0.007 | 0.499 | 6.219 | 1.847 | 4.829 | 12.651 | 0.425 | 0.785 | 3.028 | 0.035 | 10.139 | 3.754 | 0.003 | 0.121 | 0.071 | 1.094 | 5.106 | n. d.* | 1.031 | 0.793 |
| Cider-13 | 50.342 | 0.006 | 0.006 | 0.030 | 2.995 | 0.219 | 2.877 | 2.056 | 0.277 | 0.097 | 3.981 | 0.035 | 14.010 | 9.925 | 0.018 | 0.193 | 0.029 | 1.344 | 1.707 | n. d.* | 0.292 | 0.290 |
| Cider-14 | 5.086 | 0.003 | 0.010 | n. d.* | 1.505 | 0.354 | 2.501 | 0.534 | 0.304 | 0.013 | 0.235 | 0.044 | 0.502 | n. d.* | 0.006 | 0.173 | 0.014 | 0.624 | 2.649 | n. d.* | 0.841 | 0.138 |
| Cider-15 | 7.023 | 0.002 | 0.005 | 0.010 | 8.194 | 0.212 | 5.351 | 4.107 | 0.231 | 0.025 | 0.681 | 0.036 | 0.788 | n. d.* | 0.007 | 0.275 | 0.029 | 0.326 | 4.158 | n. d.* | 0.400 | 0.080 |
| Cider-16 | 6.442 | 0.004 | 0.005 | 0.004 | 4.349 | 0.239 | 8.030 | 5.120 | 0.568 | 0.025 | 0.927 | 0.133 | 0.624 | n. d.* | 0.007 | 2.403 | 0.060 | 0.915 | 11.807 | n. d.* | 1.061 | 0.162 |
| Cider-17 | 5.538 | 0.002 | 0.005 | 0.013 | 8.432 | 0.263 | 4.740 | 7.193 | 0.295 | 0.020 | 0.609 | 0.153 | 0.915 | 2.852 | 0.026 | 0.246 | 0.058 | 0.747 | 13.896 | n. d.* | 1.142 | 0.181 |
| Cider-18 | 4.715 | 0.002 | 0.005 | 0.024 | 3.180 | 0.224 | 5.718 | 5.179 | 0.412 | 0.039 | 0.672 | 0.137 | 0.758 | 3.898 | 0.031 | 0.285 | 0.031 | 0.653 | 5.913 | n. d.* | 0.748 | 0.125 |
| Cider-19 | 10.266 | 0.003 | 0.018 | 0.004 | 2.180 | 0.061 | 2.326 | n. d.* | 0.197 | 0.029 | 4.294 | 0.058 | 5.981 | 16.022 | 0.073 | 0.142 | 0.026 | 0.485 | 1.062 | n. d.* | 0.456 | 0.534 |
| Cider-20 | 18.096 | 0.003 | 0.030 | 0.021 | 2.167 | 0.062 | 1.818 | n. d.* | 0.350 | 0.021 | 3.724 | 0.061 | 6.119 | 6.823 | 0.026 | 0.212 | 0.027 | 0.637 | 0.731 | n. d.* | 0.303 | 0.179 |
| Cider-21 | 15.937 | 0.002 | 0.019 | 0.003 | 2.146 | 0.044 | 2.915 | 2.705 | 0.242 | 0.015 | 3.180 | 0.092 | 6.807 | 19.069 | 0.050 | n. d.* | 0.006 | 0.610 | 1.321 | n. d.* | 0.897 | 0.712 |
| Cider-22 | 8.744 | 0.004 | 0.022 | 0.010 | 2.911 | 0.081 | 5.224 | 2.384 | 0.310 | 0.058 | 4.650 | 0.032 | 2.036 | 10.871 | 0.011 | 0.127 | 0.011 | 1.310 | 0.914 | n. d.* | 0.366 | 0.292 |
| Cider-23 | 31.057 | 0.002 | 0.024 | 0.003 | 6.457 | 0.349 | 5.630 | 5.223 | 0.387 | 0.017 | 4.320 | 0.100 | 18.378 | 41.361 | 0.044 | 0.767 | 0.008 | 0.245 | 3.767 | n. d.* | 0.520 | 0.089 |
| Cider-24 | 21.572 | 0.003 | 0.010 | 0.008 | 7.197 | 0.333 | 8.082 | 7.334 | 0.573 | 0.019 | 4.196 | 0.227 | 11.992 | 45.493 | 0.061 | 0.581 | 0.009 | 1.313 | 4.748 | 0.001 | 1.842 | 0.319 |
| Cider-25 | 4.251 | 0.002 | 0.003 | n. d.* | 3.705 | 0.407 | 3.694 | 3.111 | 0.322 | 0.025 | 0.501 | 0.067 | 1.114 | n. d.* | 0.018 | 0.164 | 0.036 | 0.681 | 8.334 | n. d.* | 0.803 | 0.340 |
| Cider-26 | 6.707 | 0.006 | 0.049 | 0.027 | 1.141 | 0.296 | 1.809 | n. d.* | 0.431 | 0.605 | 1.963 | 0.017 | 1.833 | n. d.* | 0.006 | n. d.* | 0.076 | 0.280 | 2.218 | n. d.* | 0.474 | 0.495 |
| Cider-27 | 8.541 | 0.004 | n. d.* | 0.057 | 1.316 | 2.153 | 4.528 | 6.857 | 1.959 | 1.314 | 0.792 | 0.286 | 0.381 | 7.278 | 0.113 | n. d.* | 0.197 | 15.382 | 7.200 | 0.001 | 3.464 | 1.694 |
| Cider-28 | 7.491 | 0.005 | 0.001 | 0.013 | 6.680 | 0.634 | 11.352 | 11.724 | 1.411 | 0.343 | 2.808 | 0.460 | n. d.* | n. d.* | 0.102 | n. d.* | 0.022 | 2.829 | 4.083 | n. d.* | 3.920 | 0.980 |
| Cider-29 | 4.738 | 0.006 | 0.012 | 0.027 | 0.955 | 0.507 | 3.798 | 1.757 | 1.021 | 0.219 | 0.883 | 0.181 | n. d.* | 0.927 | 0.069 | n. d.* | 0.028 | 3.261 | 2.421 | 0.001 | 1.898 | 0.439 |
| Cider-30 | 5.581 | 0.005 | 0.056 | 0.008 | 1.089 | 0.196 | 1.212 | n. d.* | 0.276 | 0.526 | 1.331 | 0.026 | 0.269 | n. d.* | 0.002 | n. d.* | 0.041 | 0.395 | 3.200 | 0.001 | 0.672 | 0.303 |
| Cider-31 | 14.687 | 0.008 | 0.005 | 0.197 | 6.882 | 1.900 | 8.133 | 11.841 | 0.953 | 0.509 | 2.373 | 0.607 | 4.422 | 4.320 | 0.357 | n. d.* | 0.079 | 1.462 | 6.121 | 0.002 | 3.477 | 0.994 |
| Cider-32 | 24.239 | 0.002 | 0.004 | n. d.* | 1.073 | 0.208 | 1.378 | 0.564 | 0.235 | 0.004 | 0.336 | 0.075 | 5.560 | 5.933 | 0.014 | 0.249 | 0.014 | 0.265 | 3.410 | n. d.* | 0.434 | 0.242 |
| Cider-33 | 3.009 | 0.010 | 0.107 | n. d.* | 1.076 | 0.410 | 2.306 | 1.632 | 0.361 | 0.487 | 3.314 | 0.035 | 0.213 | n. d.* | 0.003 | 0.099 | 0.009 | 0.598 | 2.357 | n. d.* | 1.588 | 0.523 |
| Cider-34 | 7.651 | 0.001 | 0.004 | 0.001 | 1.588 | 0.140 | 1.781 | 0.642 | 0.151 | 0.006 | 0.297 | 0.015 | 1.626 | 3.076 | 0.001 | 0.309 | 0.010 | 0.063 | 1.865 | n. d.* | 0.120 | 0.036 |
| Compounds | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Ethyl acetate | 0.1929 | −0.2871 | 0.6272 |
| Ethyl butyrate | −0.3748 | 0.5518 | 0.1699 |
| Ethyl 2-methyl butanoate | −0.2138 | 0.7420 * | 0.4441 |
| Butyl acetate | −0.4613 | 0.4067 | 0.5826 |
| 2-Methylpropanol | −0.1353 | −0.7864 * | 0.1817 |
| Isoamyl acetate | −0.7211 * | 0.2663 | 0.2067 |
| 2-Methylbutanol | −0.3542 | −0.7724 * | 0.0937 |
| 3-Methylbutanol | −0.5470 | −0.6894 | 0.1476 |
| Ethyl hexanoate | −0.7743 * | 0.2711 | −0.0167 |
| Hexyl acetate | −0.5167 | 0.5376 | 0.4625 |
| Hexanol | 0.1405 | −0.3842 | 0.6092 |
| Ethyl octanoate | −0.7346 * | −0.4686 | −0.1391 |
| Acetic acid | 0.1652 | −0.3695 | 0.6990 |
| Butane-2,3-diol | 0.1088 | −0.2486 | 0.7352 * |
| Ethyl decanoate | −0.5897 | −0.4383 | −0.0206 |
| Diethyl succinate | 0.2157 | −0.3754 | 0.0027 |
| 2-Phenylethyl acetate | −0.6702 | 0.2028 | 0.1372 |
| Hexanoic acid | −0.6419 | −0.0540 | −0.1700 |
| 2-Phenylethanol | −0.2444 | −0.5161 | −0.1844 |
| Ethyl tetradecanoate | −0.3062 | 0.1417 | −0.2085 |
| Octanoic acid | −0.8606 * | −0.1616 | −0.1690 |
| Decanoic acid | −0.6946 | −0.0444 | −0.2195 |
| Compounds | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Ethyl acetate | −0.0112 | 0.3976 | −0.7095 * | −0.0318 |
| Ethyl butyrate | −0.4807 | −0.5699 | −0.4527 | 0.1350 |
| Ethyl 2-methyl butanoate | 0.4128 | −0.6271 | −0.3632 | 0.1209 |
| Butyl acetate | −0.8747 * | −0.3016 | −0.2470 | 0.0976 |
| 2-Methylpropanol | −0.5269 | 0.5379 | 0.2102 | 0.2558 |
| Isoamyl acetate | −0.9196 * | −0.2094 | −0.1727 | 0.1720 |
| 2-Methylbutanol | −0.6976 | 0.5118 | 0.2251 | 0.2068 |
| 3-Methylbutanol | −0.8746 * | 0.3722 | 0.1742 | −0.0042 |
| Ethyl hexanoate | −0.9127 * | −0.0729 | −0.0601 | 0.0849 |
| Hexyl acetate | −0.3249 | −0.8384 * | −0.2469 | 0.0859 |
| Hexanol | 0.0682 | 0.3553 | −0.7374 * | −0.3973 |
| Ethyl octanoate | −0.9593 * | 0.0296 | −0.1085 | 0.1515 |
| Acetic acid | −0.0189 | 0.5704 | −0.7254 * | −0.0792 |
| Butane-2,3-diol | −0.0119 | 0.6782 | −0.5551 | 0.0351 |
| Ethyl decanoate | −0.9015 * | −0.0931 | −0.2716 | −0.0397 |
| Diethyl succinate | −0.0737 | 0.3759 | 0.3839 | 0.3394 |
| 2-Phenylethyl acetate | −0.5296 | −0.5675 | 0.3575 | −0.2467 |
| Hexanoic acid | −0.2899 | 0.1588 | 0.1650 | −0.8843 * |
| 2-Phenylethanol | −0.3958 | 0.2003 | 0.7164 * | −0.3428 |
| Ethyl tetradecanoate | 0.1141 | −0.6584 | −0.1195 | 0.0137 |
| Octanoic acid | −0.9273 * | 0.0754 | −0.0263 | 0.1076 |
| Decanoic acid | −0.4703 | −0.2056 | −0.1149 | −0.7974 * |
| Code of Sample | Country of Origin * | Type of Product | ABV ** | pH | Condition of Fermentation | |||
|---|---|---|---|---|---|---|---|---|
| Autochthonous Culture | Added Yeast without Pasteurization | Pure Yeast Culture | Maturationin the Bottle | |||||
| Cider-1 | CZ | Large-scale | 4.0 | 2.87 | X | |||
| Cider-2 | CZ | Large-scale | 4.0 | 3.02 | X | |||
| Cider-3 | CZ | Large-scale | 4.0 | 2.96 | X | |||
| Cider-4 | CZ | Large-scale | 4.5 | 3.13 | X | |||
| Cider-5 | CZ | Large-scale | 5.0 | 3.08 | X | |||
| Cider-7 | CZ | Large-scale | 4.0 | 3.15 | X | |||
| Cider-10 | CZ | Small-scale | 5.5 | 3.81 | X | X | ||
| Cider-11 | CZ | Small-scale | 4.0 | 3.48 | X | |||
| Cider-12 | CZ | Small-scale | 6.0 | 3.46 | X | |||
| Cider-13 | CZ | Small-scale | 6.5 | 3.76 | X | X | ||
| Cider-27 | CZ | Small-scale | 5.0 | 3.64 | X | |||
| Cider-28 | CZ | Small-scale | 5.0 | 3.46 | X | |||
| Cider-29 | CZ | Small-scale | 5.0 | 3.90 | X | |||
| Cider-31 | CZ | Small-scale | 4.0 | 3.66 | X | |||
| Cider-8 | CZ | Small-scale | 4.9 | 3.70 | X | |||
| Cider-9 | CZ | Small-scale | 4.9 | 3.55 | X | |||
| Cider-23 | E | Small-scale | 6.0 | 3.87 | X | X | ||
| Cider-24 | E | Small-scale | 6.0 | 3.78 | X | X | ||
| Cider-32 | EE | Large-scale | 4.5 | 3.56 | X | |||
| Cider-19 | FR | Small-scale | 4.5 | 3.65 | X | X | X | |
| Cider-20 | FR | Small-scale | 5.5 | 3.68 | X | X | X | |
| Cider-21 | FR | Small-scale | 4.0 | 3.73 | X | X | X | |
| Cider-22 | FR | Small-scale | 7.0 | 3.57 | X | X | X | |
| Cider-26 | FR | Small-scale | 2.0 | 3.67 | X | X | ||
| Cider-30 | FR | Small-scale | 4.0 | 3.82 | X | X | ||
| Cider-33 | GB | Large-scale | 4.5 | 3.12 | X | |||
| Cider-34 | GB | Large-scale | 4.7 | 3.05 | X | |||
| Cider-14 | GB | Small-scale | 4.0 | 3.35 | X | |||
| Cider-15 | GB | Small-scale | 5.5 | 3.24 | X | |||
| Cider-16 | GB | Small-scale | 7.0 | 3.47 | X | |||
| Cider-17 | GB | Small-scale | 7.0 | 3.43 | X | |||
| Cider-18 | GB | Small-scale | 8.2 | 3.47 | X | |||
| Cider-25 | IE | Large-scale | 4.5 | 3.47 | X | |||
| Cider-6 | SK | Large-scale | 4.0 | 3.05 | X | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nešpor, J.; Karabín, M.; Štulíková, K.; Dostálek, P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules 2019, 24, 2117. https://doi.org/10.3390/molecules24112117
Nešpor J, Karabín M, Štulíková K, Dostálek P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules. 2019; 24(11):2117. https://doi.org/10.3390/molecules24112117
Chicago/Turabian StyleNešpor, Jakub, Marcel Karabín, Kateřina Štulíková, and Pavel Dostálek. 2019. "An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production" Molecules 24, no. 11: 2117. https://doi.org/10.3390/molecules24112117








