Hydro/Deutero Deamination of Arylazo Sulfones under Metal- and (Photo)Catalyst-Free Conditions
Abstract
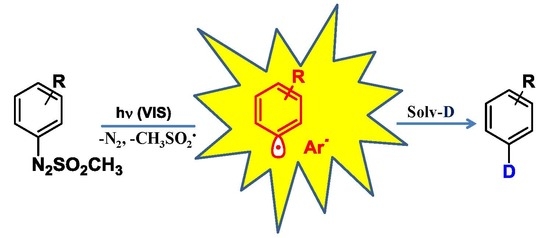
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General
4.2. General Procedure for the Synthesis of Arylazo Sulfones (1h, 1k, 1l, 1q)
4.3. General Procedure for Photochemical Irradiations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vijlder, T.; Valkenborg, D.; Lemière, F.; Romijn, E.P.; Laukens, K.; Cuyckens, F. A tutorial in small molecule identification via electrospray ionization-mass spectrometry: The practical art of structural elucidation. Mass Spectrom. Rev. 2018, 37, 607–629. [Google Scholar] [CrossRef] [PubMed]
- Guang, J.; Hopson, R.; Williard, P.G. Diffusion Coefficient-Formula Weight (D-FW) Analysis of 2H Diffusion-Ordered NMR Spectroscopy (DOSY). J. Org. Chem. 2015, 80, 9102–9107. [Google Scholar] [CrossRef] [PubMed]
- Shevlin, M.; Friedfeld, M.R.; Sheng, H.; Pierson, N.A.; Hoyt, J.M.; Campeau, L.-C.; Chirik, P.J. Nickel-Catalyzed Asymmetric Alkene Hydrogenation of α,β-Unsaturated Esters: High-Throughput Experimentation-Enabled Reaction Discovery, Optimization, and Mechanistic Elucidation. J. Am. Chem. Soc. 2016, 138, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Yayla, H.G.; Peng, F.; Mangion, I.K.; McLaughlin, M.; Campeau, L.-C.; Davies, I.W.; DiRocco, D.A.; Knowles, R.R. Discovery and mechanistic study of a photocatalytic indoline dehydrogenation for the synthesis of elbasvir. Chem. Sci. 2016, 7, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torrente, J.J.; Nguyen, D.H.; Jiménez, M.V.; Modrego, F.J.; Puerta-Oteo, R.; Gómez-Bautista, D.; Iglesias, M.; Oro, L.A. Hydrosilylation of Terminal Alkynes Catalyzed by a ONO-Pincer Iridium(III) Hydride Compound: Mechanistic Insights into the Hydrosilylation and Dehydrogenative Silylation Catalysis. Organometallics 2016, 35, 2410–2422. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Z.; Peng, X.; Sun, Y.; Ai, J.; Duan, W. Evaluation of Deuterium-Labeled JNJ38877605: Pharmacokinetic, Metabolic, and in Vivo Antitumor Profiles. Chem. Res. Toxicol. 2018, 31, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, C.; Wüst, M. Deuterium-Labeling Studies Reveal the Mechanism of Cytochrome P450-Catalyzed Formation of 2-Aminoacetophenone from 3-Methylindole (Skatole) in Porcine Liver Microsomes. J. Agric. Food Chem. 2017, 65, 10775–10780. [Google Scholar] [CrossRef]
- Parcella, K.; Eastman, K.; Yeung, K.-S.; Grant-Young, K.A.; Zhu, J.; Wang, T.; Zhang, Z.; Yin, Z.; Parker, D.; Mosure, K.; et al. Improving Metabolic Stability with Deuterium: The Discovery of BMT-052, a Pan-genotypic HCV NS5B Polymerase Inhibitor. ACS Med. Chem. Lett. 2017, 8, 771–774. [Google Scholar] [CrossRef]
- Schmidt, C. First deuterated drug approved. Nat. Biotech. 2017, 35, 493–494. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. C-H Functionalisation for Hydrogen Isotope Exchange. Angew. Chem. Int. Ed. 2018, 57, 3022–3047. [Google Scholar] [CrossRef]
- Yin, D.-W.; Liu, G. Palladium-Catalyzed Regioselective C–H Functionalization of Arenes Substituted by Two N-Heterocycles and Application in Late-Stage Functionalization. J. Org. Chem. 2018, 83, 3987–4001. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.P.; Hesk, D.; Rivera, N.; Pelczer, I.; Chirik, P.J. Iron-catalysed tritiation of pharmaceuticals. Nature 2016, 529, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Rhinehart, J.L.; Manbeck, K.A.; Buzak, S.K.; Lippa, G.M.; Brennessel, W.W.; Goldberg, K.I.; Jones, W.D. Catalytic Arene H/D Exchange with Novel Rhodium and Iridium Complexes. Organometallics 2012, 31, 1943–1952. [Google Scholar] [CrossRef]
- Pieters, G.; Taglan, C.; Bonnefill, E.; Gutmann, T.; Puente, C.; Berthet, J.-C.; Dugave, C.; Chaudret, B.; Rousseau, B. Regioselective and Stereospecific Deuteration of Bioactive Aza Compounds by the Use of Ruthenium Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.J.; Lindsay, D.M.; Owens, P.K.; Reid, M.; Tuttle, T.; Campos, S. Site-Selective Deuteration of N-Heterocycles via Iridium-Catalyzed Hydrogen Isotope Exchange. ACS Catal. 2017, 7, 7182–7186. [Google Scholar] [CrossRef] [Green Version]
- Cross, P.W.C.; Herbert, J.M.; Kerr, W.J.; McNeill, A.H.; Paterson, L.C. Isotopic Labelling of Functionalised Arenes Catalysed by Iridium(I) Species of the [(cod)Ir(NHC)(py)]PF6 Complex Class. Synlett 2016, 27, 111–115. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.-M.; Hu, Y.; Werner, T. B(C6F5)3-Catalyzed Regioselective Deuteration of Electron-Rich Aromatic and Heteroaromatic Compounds. Org. Lett. 2017, 19, 5768–5771. [Google Scholar] [CrossRef] [PubMed]
- Duttwyler, S.; Butterfield, A.M.; Siegel, J.S. Arenium Acid Catalyzed Deuteration of Aromatic Hydrocarbons. Asian J. Org. Chem. 2013, 78, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Janni, M.; Peruncheralathan, S. Catalytic selective deuteration of halo(hetero)arenes. Org. Biomol. Chem. 2016, 14, 3091–3097. [Google Scholar] [CrossRef] [Green Version]
- Oba, M. A convenient method for palladium-catalyzed reductive deuteration of organic substrates using deuterated hypophosphite in D2O. J. Label Compd. Radiopharm. 2015, 58, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-H.; Bonnesen, P.V.; Hong, K. Palladium-catalyzed Br/D exchange of arenes: Selective deuterium incorporation with versatile functional group tolerance and high efficiency. Org. Chem. Front. 2015, 2, 1071–1075. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.-H.; Schuman, D.P.; Zhong, D.; Wang, W.-Y.; Wu, L.-Y.; Liu, W.; Stoltz, B.M.; Liu, W.-B. General and Practical Potassium Methoxide/Disilane-Mediated Dehalogenative Deuteration of (Hetero)Arylhalides. J. Am. Chem. Soc. 2018, 140, 10970–10974. [Google Scholar] [CrossRef] [PubMed]
- Kallepalli, V.A.; Gore, K.A.; Shi, F.; Sanchez, L.; Chotana, G.A.; Miller, S.L.; Maleczka, R.E., Jr.; Smith, M.R., III. Harnessing C–H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization. J. Org. Chem. 2015, 80, 8341–8353. [Google Scholar] [CrossRef] [PubMed]
- Burglova, K.; Okorochenkov, S.; Hlavac, J. Efficient Route to Deuterated Aromatics by the Deamination of Anilines. Org. Lett. 2016, 18, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon-Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Fagnoni, M.; Protti, S.; Ravelli, D. (Eds.) Photoorganocatalysis in Organic Synthesis; World Scientific Publishing Europe Ltd.: Milton Keynes, UK, 2019. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Su, C.; Zhao, X.; Gao, Q.; Ning, G.-H.; Zhu, H.; Tang, W.; Leng, K.; Fu, W.; et al. Controllable deuteration of halogenated compounds by photocatalytic D2O splitting. Nat. Commun. 2018, 9, 80. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Guideline on the Specification Limits for Residues of Metal Catalysts or Metal Reagent. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-specification-limits-residues-metal-catalysts-metal-reagents_en.pdf (accessed on 21 February 2008).
- Majek, M.; Filace, F.; von Wangelin, A.J. Visible Light Driven Hydro-/Deuterodefunctionalization of Anilines. Chem. Eur. J. 2015, 21, 4518–4522. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yang, D.-Y. Visible light-mediated synthesis of quinazolines from 1,2-dihydroquinazoline 3-oxides. Tetrahedron 2013, 69, 10438–10444. [Google Scholar] [CrossRef]
- Sun, J.; He, Y.; An, X.-D.; Zhang, X.; Yu, L.; Yu, S. Visible-light-induced iminyl radical formation via electron-donor–acceptor complexes: A photocatalyst-free approach to phenanthridines and quinolines. Org. Chem. Front. 2018, 5, 977–981. [Google Scholar] [CrossRef]
- Wu, C.-K.; Yang, D.-Y. Visible-light-mediated reaction: Synthesis of quinazolinones from 1,2-dihydroquinazoline 3-oxides. RSC Adv. 2016, 6, 65988–65994. [Google Scholar] [CrossRef]
- Shi, Q.; Li, P.; Zhang, Y.; Wang, L. Visible light-induced tandem oxidative cyclization of 2-alkynylanilines with disulfides (diselenides) to 3-sulfenyl- and 3-selenylindoles under transition metal-free and photocatalyst-free conditions. Org. Chem. Front. 2017, 4, 1322–1330. [Google Scholar] [CrossRef]
- Song, L.; Zhang, L.; Luo, S.; Cheng, J.-P. Visible-Light Promoted Catalyst-Free Imidation of Arenes and Heteroarenes. Chem. Eur. J. 2014, 20, 14231–14234. [Google Scholar] [CrossRef]
- Crespi, S.; Protti, S.; Fagnoni, M. Wavelength Selective Generation of Aryl Radicals and Aryl Cations for Metal-free Photoarylations. J. Org. Chem. 2016, 81, 9612–9621. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, M.; Protti, S.; Fagnoni, M. A visible light driven, metal-free route to aromatic amides via radical arylation of isonitriles. Adv. Synth. Catal. 2017, 359, 3826–3830. [Google Scholar] [CrossRef]
- Dossena, A.; Sampaolesi, S.; Palmieri, A.; Protti, S.; Fagnoni, M. Visible light promoted metal- and photocatalyst-free synthesis of allylarenes. J. Org. Chem. 2017, 82, 10687–10692. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, L.; Raviola, C.; Di Fonzo, A.; Protti, S.; Fagnoni, M. Sunlight-driven synthesis of triarylethylenes (TAEs) via metal-free Mizoroki–Heck-type coupling. Eur. J. Org. Chem. 2018, 5297–5303. [Google Scholar] [CrossRef]
- Sauer, C.; Liu, Y.; De Nisi, A.; Protti, S.; Fagnoni, M.; Bandini, M. Photocatalyst-free, visible light driven, gold promoted Suzuki synthesis of (hetero)biaryls. ChemCatChem 2017, 9, 4456–4459. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, D.; Fagnoni, M. Wavelength-dependence and wavelength-selectivity in photochemical reactions. Photochem. Photobiol. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Liu, Y.; Song, R.-J.; Jiang, G.-F.; Li, J.-H. 1,2-Alkylarylation of Activated Alkenes with Two C–H Bonds by Using Visible-Light Catalysis. Synlett 2014, 25, 1031–1035. [Google Scholar] [CrossRef]
- Pryor, W.A.; Echols, J.T., Jr.; Smith, K. Rates of the Reactions of Substituted Phenyl Radicals with Hydrogen Donors. J. Am. Chem. Soc. 1966, 88, 1189–1199. [Google Scholar] [CrossRef]
- Kopinke, F.-D.; Zimmermann, G.; Anders, K. Relative Reactivities of C-H Bonds in H Atom Abstraction by Phenyl Radicals. J. Org. Chem. 1989, 54, 3571–3576. [Google Scholar] [CrossRef]
- Jing, L.; Guler, L.P.; Pates, G.; Kenttämaa, H.I. The Selectivity of Charged Phenyl Radicals in Hydrogen Atom Abstraction Reactions with Isopropanol. J. Phys. Chem. A 2008, 112, 9708–9715. [Google Scholar] [CrossRef] [PubMed]
- Galli, C. Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical. Chem. Rev. 1988, 88, 765–792. [Google Scholar] [CrossRef]
- Logan, C.F.; Chen, P. Ab Initio Calculation of Hydrogen Abstraction Reactions of Phenyl Radical and p-Benzyne. J. Am. Chem. Soc. 1996, 118, 2113–2114. [Google Scholar] [CrossRef]
- Luo, Y.R. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Blank, L.; Fagnoni, M.; Protti, S.; Rueping, M. Visible Light-Promoted Formation of C-B and C-S Bonds under Metal- and Photocatalyst-Free Conditions. Synthesis 2019, 51, 1243–1252. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, X.-H.; Qing, X.-H. Copper-Promoted Ritter-Type Trifluoroethoxylation of (Hetero)arenediazonium Tetrafluoroborates: A Method for the Preparation of Trifluoroethyl Imidates. Eur. J. Org. Chem. 2016, 5088–5090. [Google Scholar] [CrossRef]
- Akram, O.M.; Mali, P.S.; Patil, T.M. Cross-Coupling Reactions of Aryldiazonium Salts with Allylsilanes under Merged Gold/Visible-Light Photoredox Catalysis. Org. Lett. 2017, 19, 3075–3078. [Google Scholar] [CrossRef]
- Finger, G.C.; Oesterling, R.E. Aromatic Fluorine Compounds. VI. Displacement of Aryl Fluorine in Diazonium Salts. J. Am. Chem. Soc. 1956, 78, 2593–2596. [Google Scholar] [CrossRef]
- Ramanathan, M.; Wang, Y.-H.; Liu, Y.-H.; Peng, S.-M.; Cheng, Y.-C.; Liu, S.-T. Preparation of Ketimines from Aryldiazonium Salts, Arenes, and Nitriles via Intermolecular Arylation of N-Arylnitrilium Ions. J. Org. Chem. 2018, 83, 6133–6141. [Google Scholar] [CrossRef] [PubMed]
- Mutsumi, H.; Iwata, H.; Maruhashi, K.; Monguchi, Y.; Sajiki, H. Halogen–deuterium exchange reaction mediated by tributyltin hydride using THF-d8 as the deuterium source. Tetrahedron 2011, 67, 1158–1165. [Google Scholar] [CrossRef]
- Hanson, P.; Hendrickx, R.A.A.J.; Smith, J.R.L. An investigation by means of correlation analysis into the mechanisms of oxidation of aryl methyl sulfides and sulfoxides by dimethyldioxirane in various solvents. Org. Biomol. Chem. 2008, 6, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Oka, H.; Yamano, E.; Morita, M. Convenient Deuteration of Bromo Aromatic Compounds by Reductive Debromination with Sodium Amalgam in CH3OD. J. Org. Chem. 1997, 62, 1188–1190. [Google Scholar] [CrossRef]
- 58 Bank, S.; Schepartz, A.; Giammatteo, P.; Zubieta, J. Substituent effect on the electrochemical oxidation of arylmethyl anions. 3. Effect of methyl substitution on diarylmethyl anions. J. Org. Chem. 1983, 48, 3458–3464. [Google Scholar] [CrossRef]
- 59 Berger, S.; Diehl, B.W.K. Correlation between deuterium isotope effects and 13C-NMR chemical shifts in substituted benzenes. Tetrahedron Lett. 1987, 28, 1243–1246. [Google Scholar] [CrossRef]
- 60 Discekici, E.H.; Treat, N.J.; Poelma, S.O.; Mattson, K.M.; Hudson, Z.M.; Luo, Y.; Hawker, C.J.; de Alaniz, J.R. A highly reducing metal-free photoredox catalyst: design and application in radical dehalogenations. Chem. Commun. 2015, 51, 11705–11708. [Google Scholar] [CrossRef] [Green Version]
- 61 Barthez, J.M.; Filikov, A.V.; Frederiksen, L.B.; Huguet, M.-L.; Jones, J.R.; Lu, S.-Y. Microwave-enhanced metal- and acid-catalysed hydrogen isotope exchange reactions. Can. J. Chem. 1998, 76, 726–728. [Google Scholar] [CrossRef]
- 62 Grainger, R.; Nikmal, A.; Cornella, J.; Larrosa, I. Selective deuteration of (hetero)aromatic compounds via deutero-decarboxylation of carboxylic acids. Org. Biomol. Chem. 2012, 10, 3172–3174. [Google Scholar] [CrossRef]
- 63 Nose, M.; Suzuki, H. Convenient One-pot Procedure for Converting Aryl Sulfides to Nitroaryl Sulfones. Synthesis 2002, 8, 1065–1071. [Google Scholar] [CrossRef]
- 64 Corrie, T.J.A.; Ball, L.T.; Russell, C.A.; Lloyd-Jones, G.C. Au-Catalyzed Biaryl Coupling To Generate 5- to 9-Membered Rings: Turnover-Limiting Reductive Elimination versus π-Complexation. J. Am. Chem. Soc. 2017, 139, 245–254. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Chen, Q.; Wang, L.; He, M. Nickel-catalysed CO bond reduction of 2,4,6-triaryloxy-1,3,5-triazines in 2-methyltetrahydrofuran. Chin. Chem. Lett. 2019, 30, 409–412. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1a–r are available from the authors. |


 | |
| Ar-N2SO2Me | Product, % Yield |
| 1a, Ar = 4-CH3CO-C6H4 | 2, 76, 71 b, 61 c |
| 1b, Ar = 4-CN-C6H4 | 3, 68, 54 c |
| 1c, Ar = 4-NO2-C6H4 | 4, 55 |
| 1d, Ar = 4-Cl-C6H4 | 5, 82 |
| 1e, Ar = 4-Br-C6H4 | 6, 97 |
| 1f, Ar = 4-I-C6H4 | 7, 97 |
| 1g, Ar = 4-COOMe-C6H4 | 8, 79 |
| 1h, Ar = 3-CH3CO-C6H4 | 2, 77 |
| 1i, Ar = 3-CN-C6H4 | 3, 54 |
| 1j, Ar = 2-NO2-C6H4 | 4, 41 |
| 1k, Ar = 2-Br-C6H4 | 6, 97 |
| 1l, Ar = 2-Cl, 4-F-C6H3 | 9, 57 |
| 1m, Ar = 4-Me-C6H4 | 10, 75 |
| 1n, Ar = 4-tBu-C6H4 | 11, 78, 55 c |
| 1o, Ar = 4-CH3O-C6H4 | 12, 55 |
| 1p, Ar = 2-CH3O-C6H4 | 12, 80 |
| 1q, Ar = 3,4,5-CH3O-C6H2 | 13, 89 |
| 1r, Ar = α-Naphthyl | 14, 57 |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, H.I.M.; Raviola, C.; Amin, A.A.; Mannucci, B.; Protti, S.; Fagnoni, M. Hydro/Deutero Deamination of Arylazo Sulfones under Metal- and (Photo)Catalyst-Free Conditions. Molecules 2019, 24, 2164. https://doi.org/10.3390/molecules24112164
Amin HIM, Raviola C, Amin AA, Mannucci B, Protti S, Fagnoni M. Hydro/Deutero Deamination of Arylazo Sulfones under Metal- and (Photo)Catalyst-Free Conditions. Molecules. 2019; 24(11):2164. https://doi.org/10.3390/molecules24112164
Chicago/Turabian StyleAmin, Hawraz I. M., Carlotta Raviola, Ahmed A. Amin, Barbara Mannucci, Stefano Protti, and Maurizio Fagnoni. 2019. "Hydro/Deutero Deamination of Arylazo Sulfones under Metal- and (Photo)Catalyst-Free Conditions" Molecules 24, no. 11: 2164. https://doi.org/10.3390/molecules24112164