Improved Methods for Treatment of Phytopathogenic Biofilms: Metallic Compounds as Anti-Bacterial Coatings and Fungicide Tank-Mix Partners
Abstract
:1. Introduction
2. Results
2.1. Culturing, Treatment, and Quantification of Microbial Biofilms Using the MBEC Assay® and the BEST Assay™
2.1.1. Fungal Biofilms (Sclerotinia sclerotiorum)
2.1.2. Bacterial Biofilms
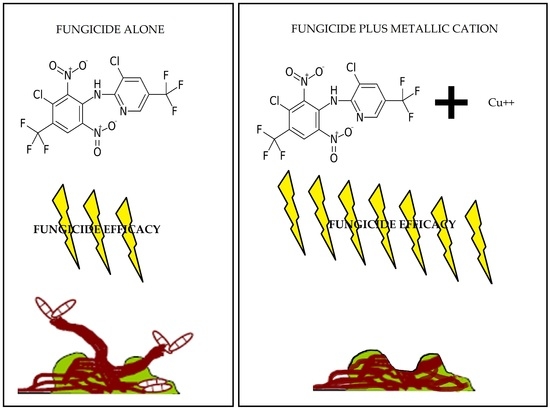
2.2. Fungicide and Metallic Cation Effects on Biofilms Formed by Bacterial Blight and White Mold Pathogens
2.2.1. Fungicides Tank Mixed with Metallic Cations versus S. sclerotiorum Biofilms.
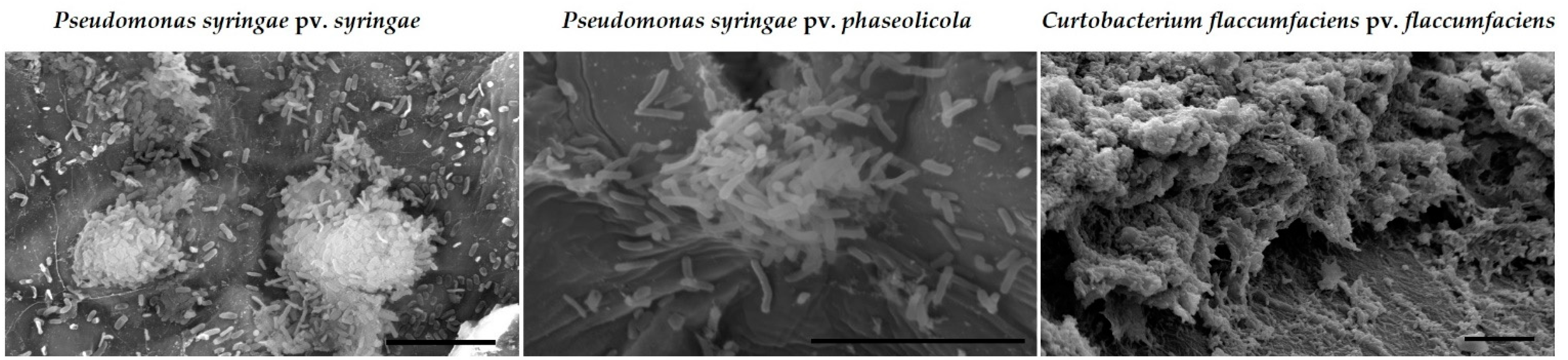
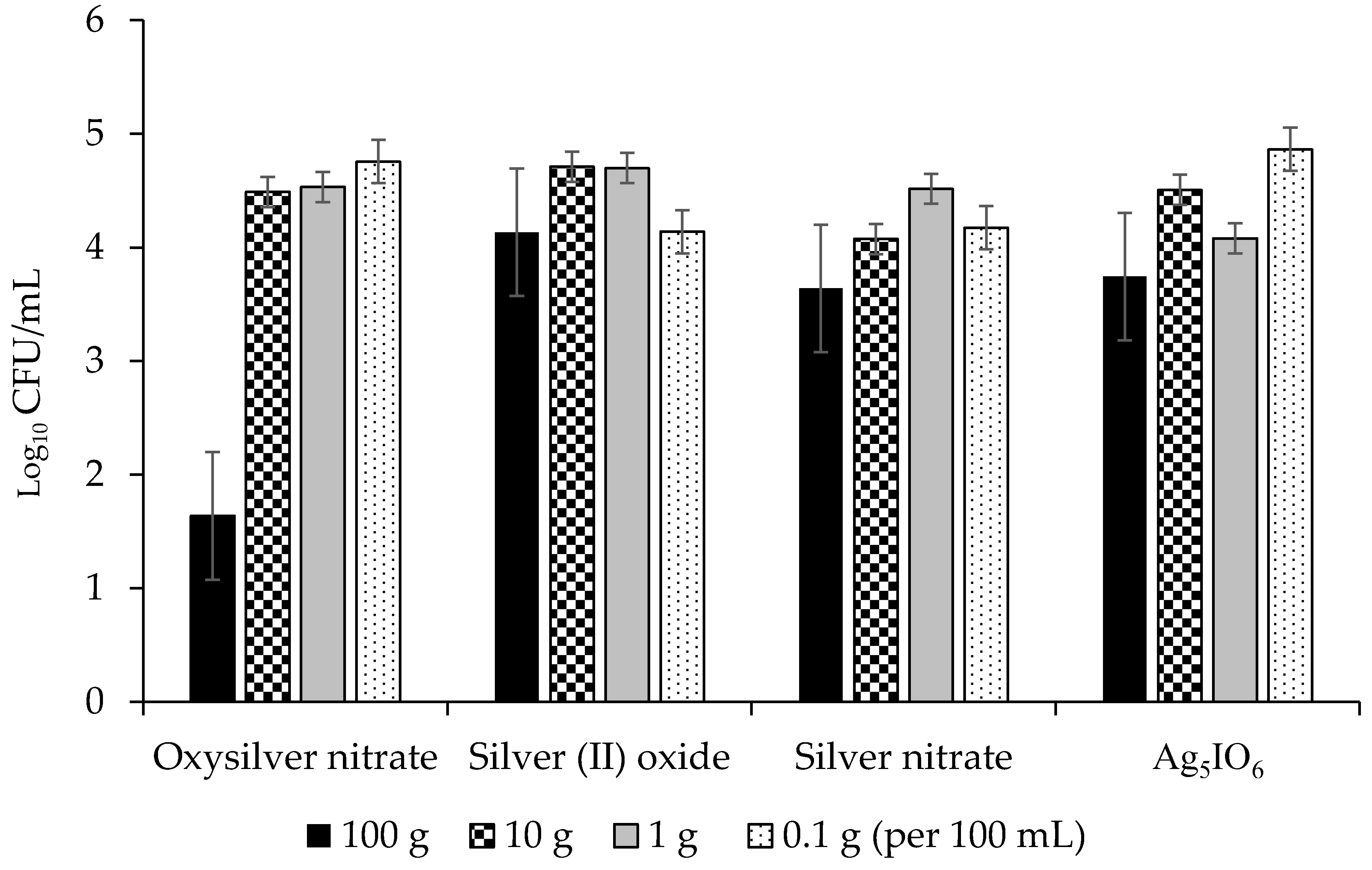
2.2.2. Abilities of Silver-Based Coatings to Reduce Adherence of Bacterial Blight Pathogens
3. Discussion
3.1. Fungicide Efficacies for Management of Sclerotinia sclerotiorum (White Mould) Biofilms
3.2. Improvement in Fungicide Efficacy via Tank Mixing Metallic Cations
3.3. Silver Coatings for Prevention of Bacterial Blight on Dry Bean Seed
3.4. General Conclusions
4. Materials and Methods
4.1. Culturing, Challenge, and Recovery of Fungal Biofilms
4.2. Testing Silver Coatings for Prevention of Bacterial Blight on Dry Bean Seed
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Verhoeven, T.L.A.; VeÂlez, M.P.; Vanderleyden, J.; De Keersmaecker, S.C.J. Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2007, 73, 6768–6775. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Kwon, M.; Oh, D.H. Impact of manganese and heme on biofilm formation of Bacillus cereus food isolates. PLoS ONE 2018, 13, e0200958. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Geesey, G.G.; Jang, L.; Jolley, J.G.; Hankins, M.R.; Iwaoka, T.; Griffiths, P.R. Binding of metal ions by extracellular polymers of biofilm bacteria. Water Sci. Technol. 1988, 20, 161–165. [Google Scholar] [CrossRef]
- Dinh, T.L.; Akhmetova, G.R.; Martykanova, D.S.; Rudakova, N.L.; Sharipova, M.R. Influence of divalent metal ions on biofilm formation by Bacillus subtilis. BioNanoSci 2019, 9, 521–527. [Google Scholar] [CrossRef]
- Polyudova, T.V.; Eroshenko, D.V.; Korobov, V.P. Plasma, serum, albumin, and divalent metal ions inhibit the adhesion and the biofilm formation of Cutibacterium (Propionibacterium) acnes. AIMS Microbiol. 2018, 4, 165–172. [Google Scholar] [CrossRef]
- Vaidya, M.Y.; McBain, A.J.; Butler, J.A.; Banks, C.E.; Whitehead, K.A. Antimicrobial efficacy and synergy of metal ions against Enterococcus faecium, Klebsiella pneumoniae and Acinetobacter baumannii in planktonic and biofilm phenotypes. Sci. Rep. 2017, 7, 5911. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.J.; Shi, Q.S.; Ouyang, Y.S.; Chen, Y.B.; Hu, W.F. Efficacy of metal ions and isothiazolones in inhibiting Enterobacter cloacae BF-17 biofilm formation. Can. J. Microbiol. 2013, 60, 5–14. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Stremick, C.A.; Turner, R.J. Biofilm susceptibility to metal toxicity. Environ. Microbiol. 2004, 6, 1220–1227. [Google Scholar] [CrossRef]
- Thornhill, S.G.; Kumar, M.; Vega, L.M.; McLean, R.J. Cadmium ion inhibition of quorum signalling in Chromobacterium violaceum. Microbiology 2017, 163, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.W.; Marques, L.L.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.W.; Marques, L.L.; Howard, R.J.; Olson, M.E. Biofilm morphologies of plant pathogenic fungi. Am. J. Plant Sci. Biotechnol. 2010, 4, 43–47. [Google Scholar]
- Rudrappa, T.; Biedrzycki, M.L.; Bais, H.P. Causes and consequences of plant-associated biofilms. FEMS Microbiol. Ecol. 2008, 64, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Bogino, P.C.; Oliva, M.L.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef]
- Li, J.; Wang, N. Foliar application of biofilm formation–inhibiting compounds enhances control of citrus canker caused by Xanthomonas citri subsp. citri. Phytopathology 2014, 104, 134–142. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal biofilms: Targets for the development of novel strategies in plant disease management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef]
- Saharan, G.S.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer Science & Business Media: Berlin, Germany, 2008; pp. 13–417. [Google Scholar]
- Mahoney, K.J.; McCreary, C.M.; Gillard, C.L. Response of dry bean white mould [Sclerotinia sclerotiorum (Lib.) de Bary, causal organism] to fungicides. Can. J. Plant Sci. 2014, 94, 905–910. [Google Scholar] [CrossRef]
- Harveson, R.M.; Schwartz, H.F. Bacterial diseases of dry edible beans in the Central High Plains. Plant Health Prog. 2007, 8, 35. [Google Scholar] [CrossRef]
- Ramasubramaniam, H.; del Río Mendoza, L.E.; Bradley, C.A. Estimates of yield and economic losses associated with white mold of rain-fed dry bean in North Dakota. Agron. J. 2008, 100, 315–319. [Google Scholar] [CrossRef]
- Anwar, H.; Dasgupta, M.K.; Costerton, J.W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother. 1990, 34, 2043–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, C.; Duan, H.; Bi, Y.; Wu, D.; Du, J.; Yu, H. Synergistic Antifungal Effect of Biosynthesized Silver Nanoparticles Combined with Fungicides. Int. J. Agric. Biol. 2018, 20, 1225–1229. [Google Scholar]
- Derbyshire, M.C.; Denton-Giles, M. The control of sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef]
- PoSlušná, J.; Plachká, E.; Mazáková, J. Influence of selected fungicides registered in the Czech Republic for winter oilseed rape on in vitro Sclerotinia sclerotiorum mycelial growth. Plant Prot. Sci. 2018, 54, 101–110. [Google Scholar]
- Mueller, D.S.; Dorrance, A.E.; Derksen, R.C.; Ozkan, E.; Kurle, J.E.; Grau, C.R.; Gaska, J.M.; Hartman, G.L.; Bradley, C.A.; Pedersen, W.L. Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Dis. 2002, 86, 26–31. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Activity of boscalid, fenhexamid, fluazinam, fludioxonil, and vinclozolin on growth of Sclerotinia minor and S. sclerotiorum and development of lettuce drop. Plant Dis. 2004, 88, 665–668. [Google Scholar] [CrossRef]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef]
- Hussain, S.S.; Al-Nuzal, S.M.; Al-Qazzaz, S.F. Preparation of copper oxalate and copper oxide nanoparticles and their antibacterial effect against P. aeruginosa and methicillin resistant S. aureus from burn and wound infections. J. Gen. Environ. Res. Conserv. 2015, 3, 173–187. [Google Scholar]
- Mhatre, E.; Troszok, A.; Gallegos-Monterrosa, R.; Lindstädt, S.; Hölscher, T.; Kuipers, O.P.; Kovács, Á.T. The impact of manganese on biofilm development of Bacillus subtilis. Microbiology 2016, 162, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, E.; Sundaram, A.; Hölscher, T.; Mühlstädt, M.; Bossert, J.; Kovács, Á. Presence of calcium lowers the expansion of Bacillus subtilis colony biofilms. Microorganisms 2017, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, B.; Saucedo-Mora, M.Á.; Roldán-Sánchez, J.A.; Pérez-Eretza, B.; Ramasamy, M.; Lee, J.; Coria-Jimenez, R.; Tapia, M.; Varela-Guerrero, V.; García-Contreras, R. Inhibition of quorum-sensing-dependent virulence factors and biofilm formation of clinical and environmental P seudomonas aeruginosa strains by ZnO nanoparticles. Lett. Appl. Microbiol. 2015, 61, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Young, D.H.; Avila-Adame, C.; Webster, J.; Olson, B.; Ouimette, D. Enhancing the efficacy of copper fungicides through synergism with salicylaldehyde benzoylhydrazones. In Modern Fungicides and Antifungal Compounds Vol III.; Deising, H.B., Fraaije, B., Mehl, A., Oerke, E.C., Sierotzki, H., Stammler, G., Eds.; Deutsche Phytomedizinische Gesellschaf: Braunschweig, Germany, 2017; Volume VIII, pp. 273–278. [Google Scholar]
- Li, J.; Liu, H.; Guo, Z.; Yang, M.; Song, J.; Ma, H. Two Cu (ii)-triadimenol complexes as potential fungicides: Synergistic actions and DFT calculations. RSC Adv. 2018, 8, 2933–2940. [Google Scholar] [CrossRef]
- Uivarosi, V. Metal complexes of quinolone antibiotics and their applications: An update. Molecules 2013, 18, 11153–11197. [Google Scholar] [CrossRef] [PubMed]
- Kataria, H.R.; Sunder, S. Effect of micronutrients on the efficacy of fungicides against Rhizoctonia solani on cowpea seedlings. Pestic. Sci. 1985, 16, 453–456. [Google Scholar] [CrossRef]
- Kurt, S.; Dervis, S.; Sahinler, S. Sensitivity of Verticillium dahlia to prochloraz and prochloraz-managanese complex and control of Verticillium wilt of cotton in the field. Crop. Prot. 2003, 22, 51–55. [Google Scholar] [CrossRef]
- Campagna, G.; Brignoli, P. The use of coadjuvants in tank mix with fungicides in order to improve their effectiveness even at low dosages. J. Cent. Eur. Agric. 2005, 6, 603–610. [Google Scholar]
- Amaradasa, B.S.; Everhart, S.E. Effects of sublethal fungicides on mutation rates and genomic variation in fungal plant pathogen, Sclerotinia sclerotiorum. PLoS ONE 2016, 11, e0168079. [Google Scholar] [CrossRef]
- Chatterton, S.; Balasubramanian, P.M.; Erickson, R.S.; Hou, A.; McLaren, D.L.; Henriquez, M.A.; Conner, R.L. Identification of bacterial pathogens and races of Pseudomonas syringae pv. phaseolicola from dry bean fields in Western Canada. Can. J. Plant Pathol. 2016, 38, 41–54. [Google Scholar]
- Harding, M.W.; Daniels, G.C. In vitro assessment of biofilm formation by soil and plant associated microorganisms. In Biofilms in Plant and Soil Health; Ahmad, I., Husain, F.M., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2017; pp. 253–273. [Google Scholar]
- Harding, M.W.; Olson, M.E.; Marques, L.L.R.; Howard, R.J. Biology and management of microbial biofilms on plant surfaces. In ISHS Acta Horticulturae 905: International Symposium on Biological Control of Postharvest Diseases: Challenges and Opportunities; Wisniewski, M., Droby, S., Eds.; ISHS: Leuven, Belgium, 2011; pp. 43–56. [Google Scholar]
Sample Availability: Not available. |





| Fungicide | Metal | Lg Reduction (CFU/mL) |
|---|---|---|
| Fluazinam | Cu2+ | 2.18 a |
| Boscalid | Ag+ | 1.66 ab |
| Fludioxonil | Ag+ | 1.59 ab |
| Cyprodinil | Cu2+ | 1.58 abc |
| Fluazinam | Ag+ | 1.35 abcd |
| Fludioxonil | Cu2+ | 1.30 abcde |
| Boscalid | Cu2+ | 1.23 bcde |
| Cyprodinil | Ca2+ | 1.23 bcde |
| Cyprodinil | Zn2+ | 1.21 bcde |
| Cyprodinil | Mn2+ | 1.17 bcdef |
| Boscalid | B+ | 1.13 bcdefg |
| Boscalid | Ca2+ | 1.06 bcdefg |
| Cyprodinil | B+ | 1.05 bcdefg |
| Cyprodinil | Ag+ | 1.05 bcdefg |
| Boscalid | None | 0.96 bcdefg |
| Fluazinam | Zn2+ | 0.85 bcdefg |
| Boscalid | Mn2+ | 0.78 bcdefg |
| Boscalid | Zn2+ | 0.72 bcdefg |
| Fluazinam | None | 0.64 cdefg |
| Fluazinam | Ca2+ | 0.62 defg |
| Fluazinam | Zn2+ | 0.56 defg |
| Fluazinam | B+ | 0.54 defg |
| Cyprodinil | None | 0.44 defg |
| Fludioxonil | Mn2+ | 0.43 defg |
| Fludioxonil | Ca2+ | 0.42 defg |
| Fludioxonil | B+ | 0.37 efg |
| Fludioxonil | None | 0.25 fg |
| Fludioxonil | Zn2+ | 0.19 g |
| Silver Coating | PSS 1 Growth (lg CFU/mL) | PSP 2 Growth (lg CFU/mL) | CFF 3 Growth (lg CFU/mL) |
|---|---|---|---|
| Oxysilver nitrate | 4.26 a | 5.27 a | 3.85 a |
| Silver (II) oxide | 4.75 ab | 6.22 b | 4.42 b |
| Ag5IO6 | 5.05 ab | 6.10 b | 4.10 b |
| Silver nitrate | 5.1 b | 6.01 b | 4.30 b |
| Compound | Source | Concentrations (g/L) |
|---|---|---|
| Boscalid | 2-chloro-N-[2-(4-chlorophenyl)phenyl]pyridine-3-carboxamide | 4.9, 2.7, 1.79 |
| Cyprodinil | 4-cyclopropyl-6-methyl-N-phenylpyrimidin-2-amine | 2.74, 1.83, 1.0 |
| Fluazinam | 3-chloro-N-[3-chloro-2,6-dinitro-4-(trifluoromethyl)phenyl]-5-(trifluoromethyl)pyridin-2-amine | 4.55, 3.03, 1.67 |
| Fludioxonil | 4-(2,2-difluoro-1,3-benzodioxol-4-yl)-1H-pyrrole-3-carbonitrile | 1.83, 1.22, 0.67 |
| Penthiopyrad | 1-methyl-N-[2-(4-methylpentan-2-yl)thiophen-3-yl]-3-(trifluoromethyl)pyrazole-4-carboxamide | 2.72, 1.49, 0.99 |
| Picoxystrobin | methyl (E)-3-methoxy-2-[2-[[6-(trifluoromethyl)pyridin-2-yl]oxymethyl]phenyl]prop-2-enoate | 2.0, 1.1, 0.73 |
| Ca2+ | Ca(NO3)2 | 0.029, 0.015, 0.0096 |
| B+ | Na2[B4O5(OH)4] | 3.05,1.68, 1.12 |
| Cu2+ | CuSO4, | 3.05, 1.68, 1.12 |
| Mn2+ | MnSO4 | 3.05, 1.68, 1.12 |
| Ag+ | AgNO3 | 4.55, 2.5, 1.67 |
| Zn2+ | ZnSO4 | 5.09, 2.8, 1.86 |
| Compound | Formula | Concentrations * (mg/1.5 mL) |
|---|---|---|
| Oxysilver nitrate | Ag(Ag2O4)2NO3 | 150, 15, 1.5, 0.15 |
| Silver (II) oxide | AgO | 138, 13.8, 1.38, 0.138 |
| Silver nitrate | AgNO3 | 189, 18.9, 1.89, 0.189 |
| Pentasilver hexaoxoiodate | Ag5IO6 | 169, 16.9, 1.69, 0.169 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harding, M.; Nadworny, P.; Buziak, B.; Omar, A.; Daniels, G.; Feng, J. Improved Methods for Treatment of Phytopathogenic Biofilms: Metallic Compounds as Anti-Bacterial Coatings and Fungicide Tank-Mix Partners. Molecules 2019, 24, 2312. https://doi.org/10.3390/molecules24122312
Harding M, Nadworny P, Buziak B, Omar A, Daniels G, Feng J. Improved Methods for Treatment of Phytopathogenic Biofilms: Metallic Compounds as Anti-Bacterial Coatings and Fungicide Tank-Mix Partners. Molecules. 2019; 24(12):2312. https://doi.org/10.3390/molecules24122312
Chicago/Turabian StyleHarding, Michael, Patricia Nadworny, Brenton Buziak, Amin Omar, Greg Daniels, and Jie Feng. 2019. "Improved Methods for Treatment of Phytopathogenic Biofilms: Metallic Compounds as Anti-Bacterial Coatings and Fungicide Tank-Mix Partners" Molecules 24, no. 12: 2312. https://doi.org/10.3390/molecules24122312