The Role of Orientation of Surface Bound Dihydropyrrol-2-ones (DHP) on Biological Activity
Abstract
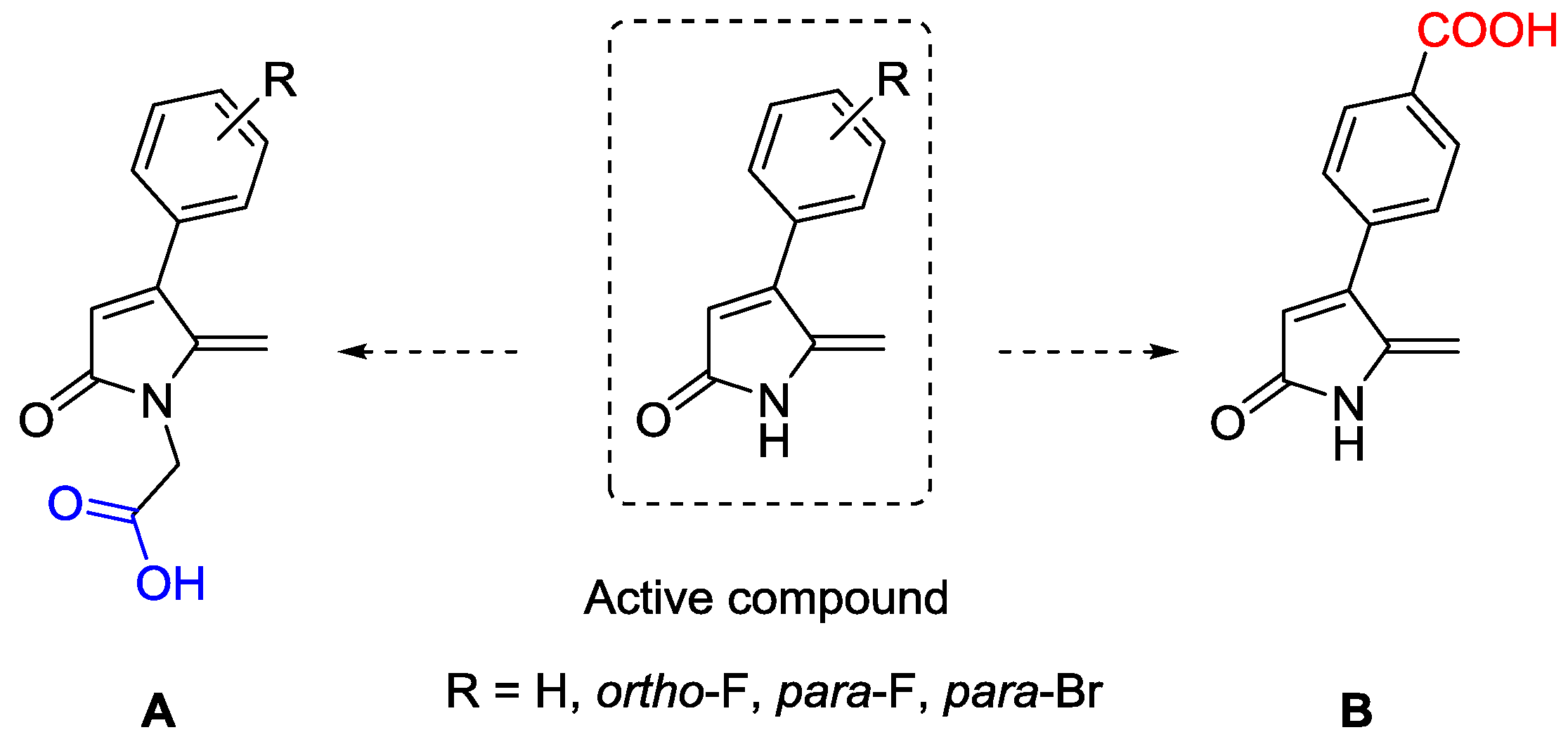
:1. Introduction
2. Results
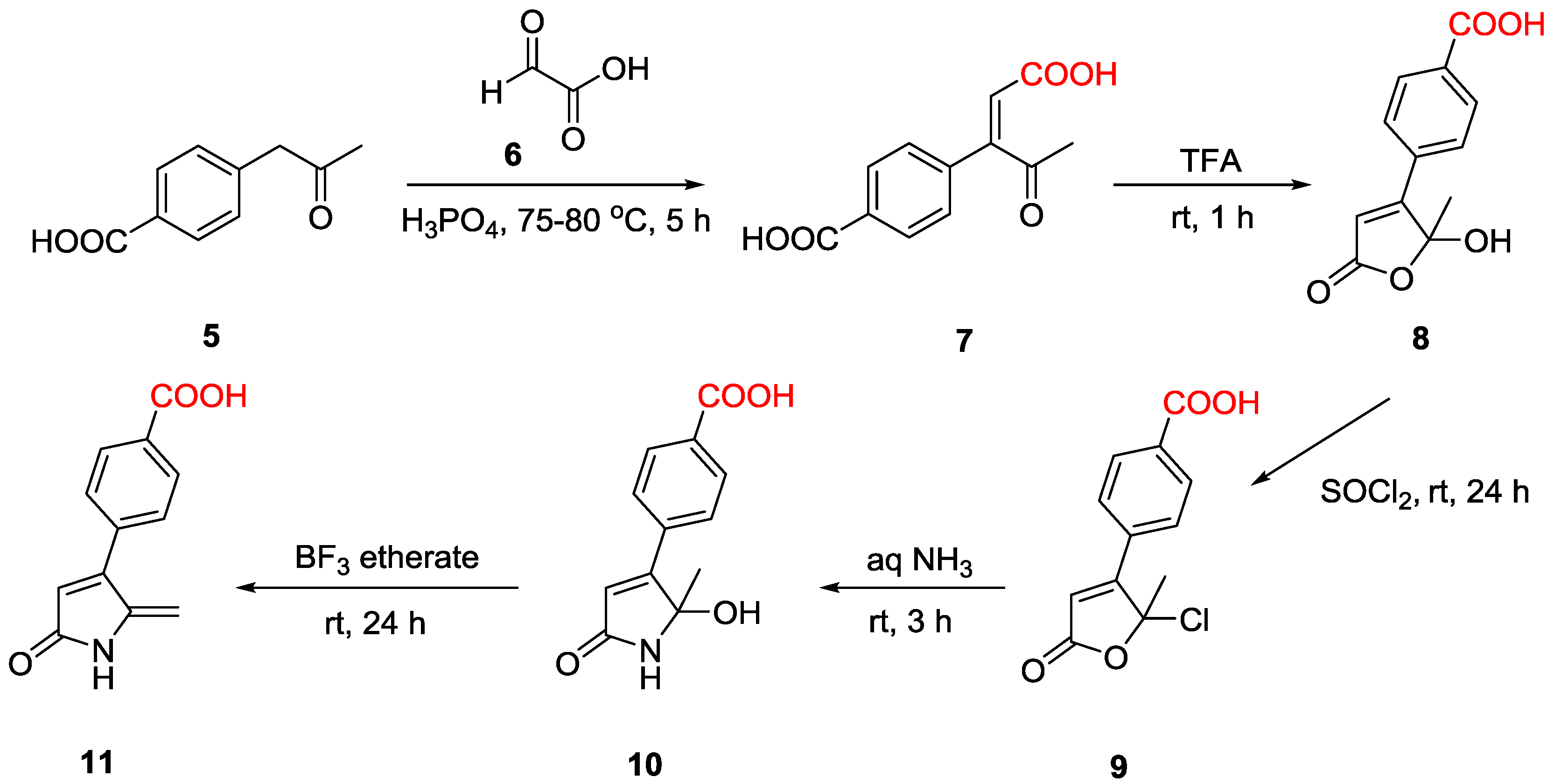
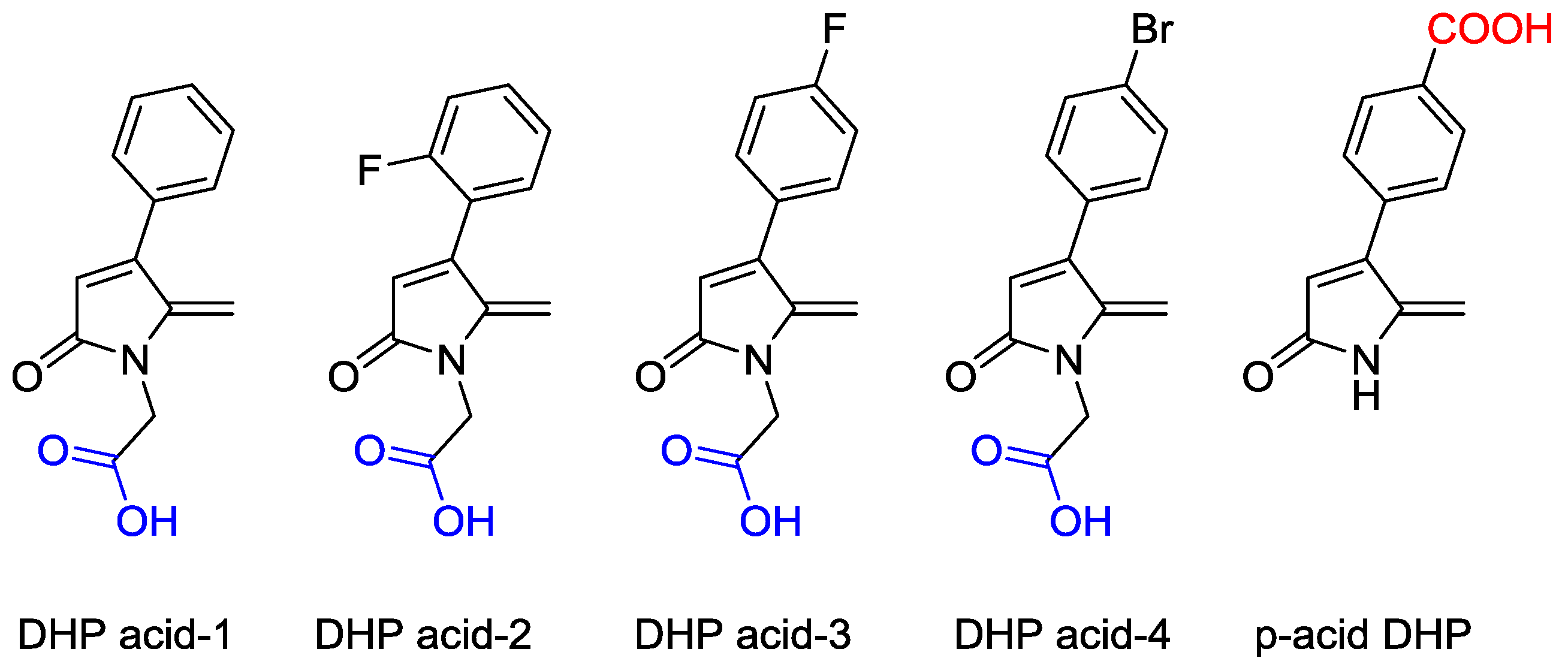
2.1. Synthesis of DHP Analogues
2.2. Surface Characterization by X-ray Photoelectron Spectroscopy (XPS)
2.3. Contact Angle Measurements
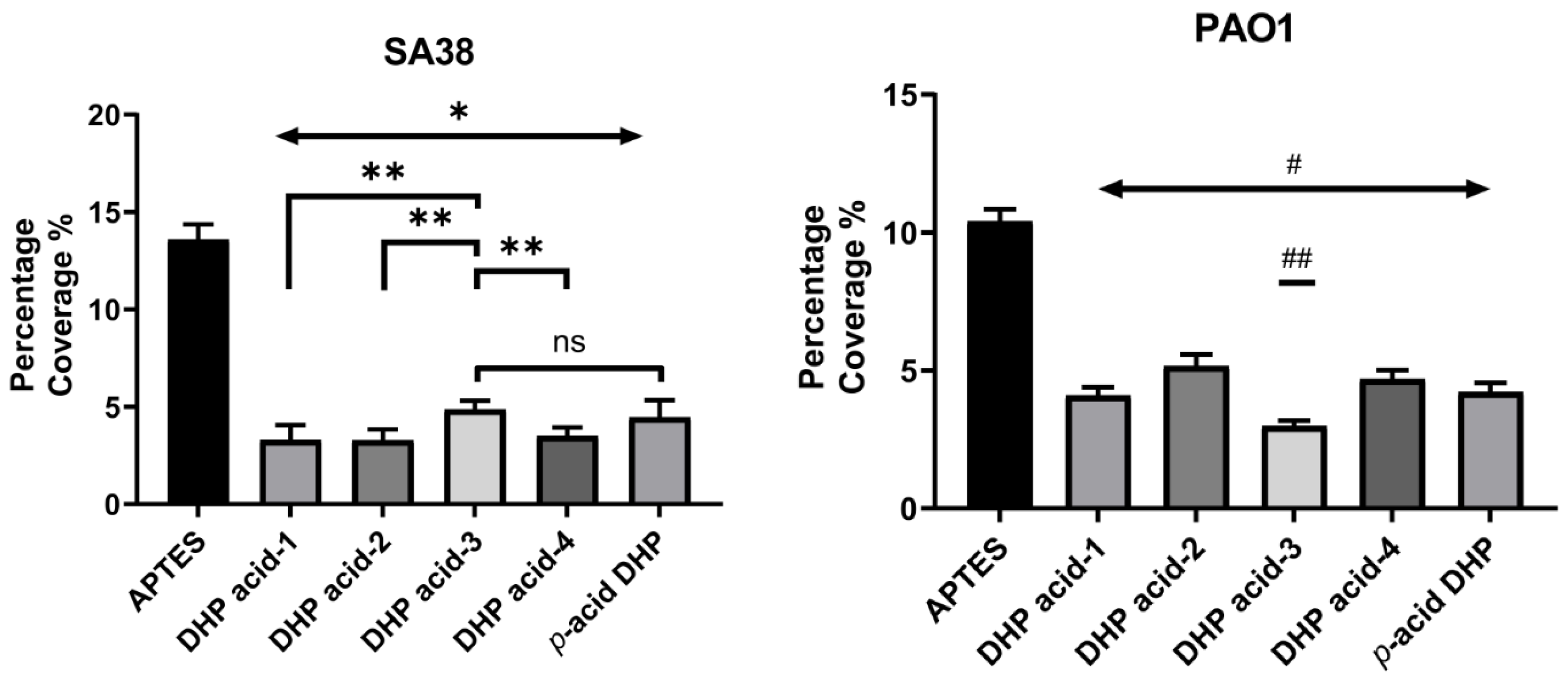
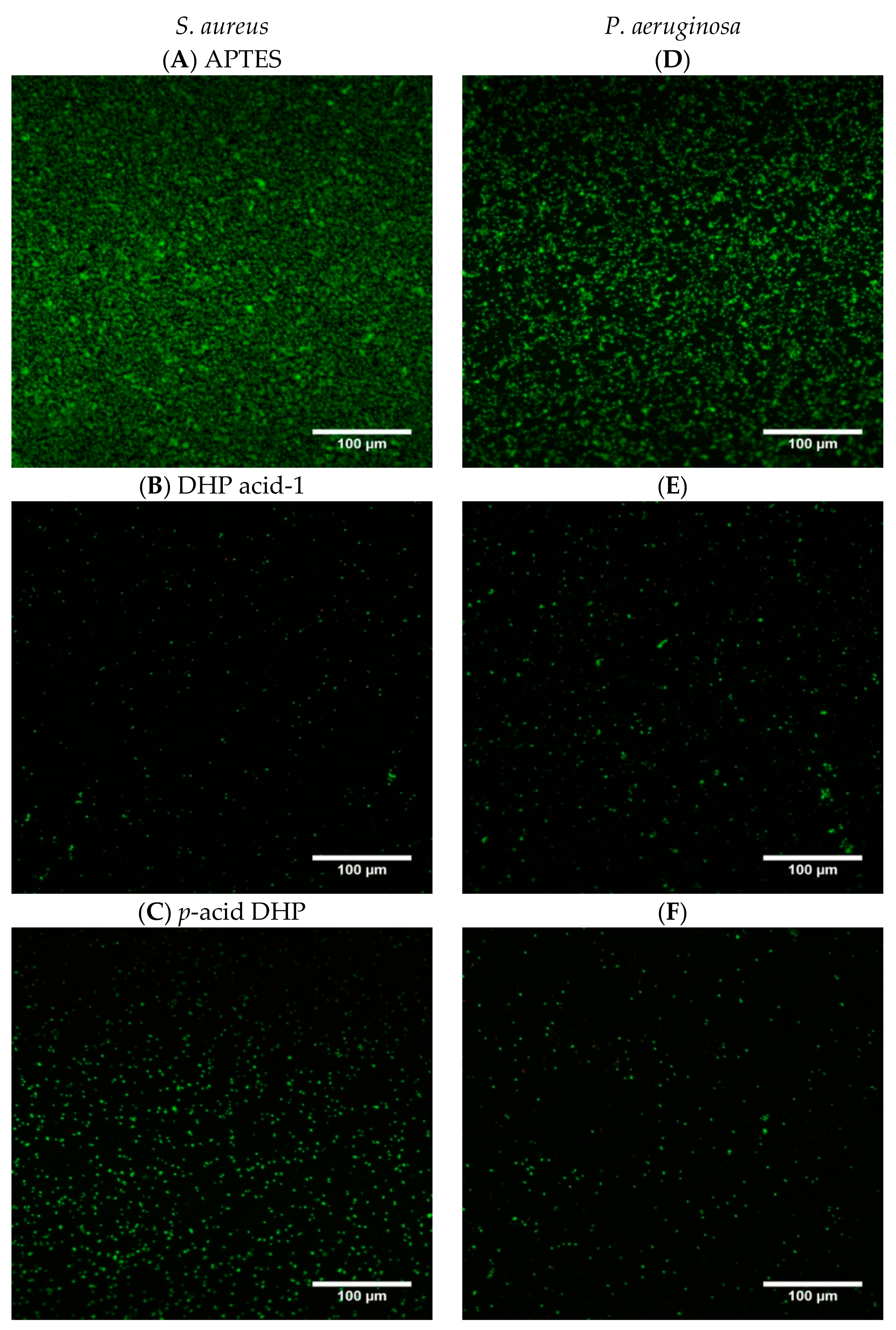
2.4. Antibacterial Activity
2.5. Quorum Sensing Inhibition Assay
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of DHP Derivatives
4.2.1. Tert-Butyl-2-(5-(2-(tert-butoxy)-2-oxoethoxy)-5-methyl-2-oxo-4-phenyl-1,5-dihydro-2H-pyrrol-1-yl) acetate (3, R = H)
4.2.2. 2-(5-Methylene-2-oxo-4-phenyl-1,5-dihydro-2H-pyrrol-1-yl)acetic acid (DHP acid-1)
4.2.3. 2-(5-Methylene-2-oxo-4-(2-fluorophenyl)-1,5-dihydro-2H-pyrrol-1-yl)acetic acid (DHP acid-2)
4.2.4. 2-(5-Methylene-2-oxo-4-(4-fluorophenyl)-1,5-dihydro-2H-pyrrol-1-yl)acetic acid (DHP acid-3)
4.2.5. 2-(5-Methylene-2-oxo-4-(4-bromophenyl)-1,5-dihydro-2H-pyrrol-1-yl)acetic acid (DHP acid-4)
4.2.6. (Z)-4-(1-Carboxy-3-oxobut-1-en-2-yl)benzoic acid 7
4.2.7. 5-Hydroxy-5-methyl-4-(4-carboxyphenyl)-1,5-dihydro-2H-pyrrol-2-one 10
4.2.8. 4-(4-Carboxy phenyl)-5-methyelene-1,5-dihydro-2H-pyrrol-2-one (p-acid DHP) 11
4.3. Attachment of (3-Aminopropyl)triethoxysilane (APTES)
4.4. Attachment of DHPs
4.5. X-ray Photoelectron Spectroscopy
4.6. Contact Angle Measurements
4.7. Bacterial Adhesion Analysis
4.8. Statistical Analysis of Data
4.9. Quorum Sensing Inhibition Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The World Health Report 2000–Health Systems: Improving Performance; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Gatermann, S.; Funfstuck, R.; Handrick, W.; Leitritz, L.; Naber, K.; Podbielski, A.; Schmidt, H.; Sester, U.; Straube, E.; Wittke, J.W. MIQ 02: Urinary Tract Infections: Quality Standards for Microbiological Infections; Urban & Fischer: München, Germany, 2005; pp. 8–21. [Google Scholar]
- US Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; US Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016, pp. 1–40. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 20 June 2019).
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance. 2014, pp. 1–16. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 20 June 2019).
- Baddour, L.M.; Cha, Y.-M.; Wilson, W.R. Infections of Cardiovascular Implantable Electronic Devices. N. Engl. J. Med. 2012, 367, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciorba, A.; Bovo, R.; Trevisi, P.; Rosignoli, M.; Aimoni, C.; Castiglione, A.; Martini, A. Postoperative complications in cochlear implants: A retrospective analysis of 438 consecutive cases. Eur. Arch. Otorhinolaryngol. 2012, 269, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Muench, P.J. Infections versus penile implants: The war on bugs. J. Urol. 2013, 189, 1631–1637. [Google Scholar] [CrossRef]
- Mermel, L.A. Prevention of Instravascular Catheter-Related Infections. Ann. Intern. Med. 2000, 132, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. Correction: Catheter-Related Bloodstream Infections. Ann. Intern. Med. 2000, 133, 395. [Google Scholar]
- Stickler, D.J. Susceptibility of antibiotic-resistant gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J. Appl. Microbiol. Symp. Suppl. 2002, 92, 163S–170S. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Teimory, M.; Tabandeh, H.; Kirwan, J.F.; Dalton, R.; Reid, F.; Rostron, C.K. Intraocular lens implants and risk of endophthalmitis. Br. J. Ophthalmol. 1998, 82, 1312–1315. [Google Scholar] [CrossRef] [Green Version]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006, 35, 780. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.S.; Robertson, G.T. Bacterial and fungal biofilm infections. Annu. Rev. Med. 2008, 59, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; Van den Dungen, J.J.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Iskander, G. Furanone Compounds and Lactam Analogues Thereof. U.S. Patent No. 8,586,618, 19 November 2013. [Google Scholar]
- Kumar, N.; Iskander, G. Novel Lactams 2007. WO 2007/085042 A1, 7 August 2014. [Google Scholar]
- Ho, K.K.K.; Cole, N.; Chen, R.; Willcox, M.D.P.; Rice, S.A.; Kumar, N. Immobilization of antibacterial dihydropyrrol-2-ones on functional polymer supports to prevent bacterial infections in vivo. Antimicrob. Agents Chemother. 2012, 56, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.K.; Chen, R.; Willcox, M.D.P.; Rice, S.A.; Cole, N.; Iskander, G.; Kumar, N. Quorum sensing inhibitory activities of surface immobilized antibacterial dihydropyrrolones via click chemistry. Biomaterials 2014, 35, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Ka Kit Ho, K.; Glattauer, V.; Willcox, M.; Kumar, N.; Thissen, H. Poly(ethylene glycol)-Based Coatings Combining Low-Biofouling and Quorum-Sensing Inhibiting Properties to Reduce Bacterial Colonization. ACS Biomater. Sci. Eng. 2016, 3, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Taunk, A.; Ho, K.K.K.; Iskander, G.; Willcox, M.D.P.; Kumar, N. Surface immobilization of antibacterial quorum sensing inhibitors by photochemical activation. J. Biotechnol. Biomater. 2016, 6, 1000238. [Google Scholar] [CrossRef]
- Almohaywi, B.; Yu, T.T.; Iskander, G.; Chan, D.S.H.; Ho, K.K.K.; Rice, S.; Black, D.S.C.; Griffith, R.; Kumar, N. Dihydropyrrolones as bacterial quorum sensing inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 1054–1059. [Google Scholar] [CrossRef]
- Ho, K.K.K.; Cole, N.; Chen, R.; Willcox, M.D.P.; Rice, S.A.; Kumar, N. Characterisation and in vitro activities of surface attached dihydropyrrol-2-ones against Gram-negative and Gram-positive bacteria. Biofouling 2010, 26, 913–921. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface modification by allylamine plasma polymerization promotes osteogenic differentiation of human adipose-derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef]
- López-Pérez, P.M.; Marques, A.P.; Da Silva, R.M.P.; Pashkuleva, I.; Reis, R.L. Effect of chitosan membrane surface modification via plasma induced polymerization on the adhesion of osteoblast-like cells. J. Mater. Chem. 2007, 17, 4064–4071. [Google Scholar] [CrossRef] [Green Version]
- Tenover, F.C. Development and spread of bacterial resistance to antimicrobial agents: an overview. Clin. Infect. Dis. 2001, 33, S108–S115. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Hoiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flipo, M.; Desroses, M.; Lecat-Guillet, N.; Villemagne, B.; Blondiaux, N.; Leroux, F.; Piveteau, C.; Mathys, V.; Flament, M.P.; Siepmann, J.; et al. Ethionamide boosters. 2. Combining bioisosteric replacement and structure-based drug design to solve pharmacokinetic issues in a series of potent 1,2,4-oxadiazole EthR inhibitors. J. Med. Chem. 2012, 55, 68–83. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef]
- Phillips, G.J. Green fuorescent protein—A bright idea for the study of bacterial protein localization. FEMS Microbiol. Lett. 2001, 204, 9–18. [Google Scholar]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef] [Green Version]
- Qazi, S.; Middleton, B.; Muharram, S.H.; Cockayne, A.; Hill, P.; O’Shea, P.; Chhabra, S.R.; Cámara, M.; Williams, P. N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect. Immun. 2006, 74, 910–919. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, G.M.; Van der Mei, H.C.; Busscher, H.J. Bactetial adhesion to surface hydrophlic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Silva, L.N.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Escherichia coli adhesion, biofilm development and antibiotic susceptibility on biomedical materials. J. Biomed. Mater. Res. Part A 2015, 103, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Bendinger, B.; Rijnaarts, H.H.M.; Altendorf, K.; Zehnder, A.J.B. Physicochemical cell-surface and adhesive properties of coryneform bacteria related to the presence and chain-length of mycolic acids. Appl. Environ. Microbiol. 1993, 59, 3973–3977. [Google Scholar] [PubMed]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.P.; Cole, N.; Ho, K.K.K.; Rasul, R.; Denman, J.A.; Kumar, N. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012, 8, 4371–4379. [Google Scholar] [CrossRef]
- Taunk, A.; Chen, R.; Iskander, G.; Ho, K.K.K.; Black, D.S.C.; Willcox, M.D.P.; Kumar, N. Dual-Action Biomaterial Surfaces with Quorum Sensing Inhibitor and Nitric Oxide to Reduce Bacterial Colonization. ACS Biomater. Sci. Eng. 2018, 4, 4174–4182. [Google Scholar] [CrossRef]
- Ciampi, S.; Böcking, T.; Kilian, K.A.; James, M.; Harper, J.B.; Gooding, J.J. Functionalization of acetylene-terminated monolayers on Si(100) surfaces: A click chemistry approach. Langmuir 2007, 23, 9320–9329. [Google Scholar] [CrossRef]
- Kirov, S.M.; Webb, J.S.; O’May, C.Y.; Reid, D.W.; Woo, J.K.K.; Rice, S.A.; Kjelleberg, S. Biofilm differentiation and dispersal in mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 2007, 153, 3264–3274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barraud, N.; Hassett, D.J.; Hwang, S.-H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Surface | %C | %N | %Halogen |
|---|---|---|---|
| Blank | 6.6 | 0.6 | - |
| APTES | 45.4 | 8.1 | - |
| DHP acid-1 | 48.6 | 8.9 | - |
| DHP acid-2 | 49.3 | 8.8 | 0.40% F |
| DHP acid-3 | 47.2 | 10.8 | 0.17% F |
| DHP acid-4 | 51.7 | 10.3 | 0.91% Br |
| p-acid DHP | 50.2 | 9.2 | - |
| Surface | C 1s | N 1s | ||||
|---|---|---|---|---|---|---|
| Binding energy (eV) | Assignment | Peak area (%) | Binding energy (eV) | Assignment | Peak area (%) | |
| APTES | 284.9 | C-C | 63.0 | 399.5 | NH2 | 80.2 |
| 285.9 | C-N | 25.5 | 401.4 | NH3+, Tertiary N | 19.7 | |
| 287.9 | C=O | 11.4 | ||||
| DHP acid-1 | 284.8 | C-C | 57.0 | 399.5 | NH2 | 34.0 |
| 285.8 | C-N | 21.0 | 400.2 | N-C=O | 56.6 | |
| 287.8 | C=O | 11.6 | 401.6 | NH3+, Tertiary N | 9.4 | |
| 288.6 | N-C=O | 10.4 | ||||
| DHP acid-2 | 284.8 | C-C | 56.7 | 399.5 | NH2 | 30.9 |
| 285.8 | C-N | 21.2 | 400.2 | N-C=O | 58.3 | |
| 287.8 | C=O | 12.1 | 401.6 | NH3+, Tertiary N | 10.8 | |
| 288.6 | N-C=O | 10.0 | ||||
| DHP acid-3 | 284.8 | C-C | 55.8 | 399.4 | NH2 | 21.7 |
| 285.8 | C-N | 24.2 | 400.0 | N-C=O | 67.6 | |
| 287.8 | C=O | 10.0 | 401.6 | NH3+, Tertiary N | 10.7 | |
| 288.7 | N-C=O | 10.0 | ||||
| DHP acid-4 | 284.8 | C-C | 60.0 | 399.4 | NH2 | 36.8 |
| 285.8 | C-N | 22.8 | 400.0 | N-C=O | 59.7 | |
| 287.6 | C=O | 8.8 | 401.4 | NH3+, Tertiary N | 3.5 | |
| 288.6 | N-C=O | 8.4 | ||||
| p-acid DHP | 284.8 | C-C | 67.2 | 399.6 | NH2 | 61.6 |
| 285.8 | C-N | 12.0 | 400.1 | N-C=O | 31.3 | |
| 287.9 | C=O | 14.1 | 401.5 | NH3+, Tertiary N | 7.1 | |
| 288.4 | N-C=O | 6.6 | ||||
| Surface | Contact Angle (°) (±1) |
|---|---|
| Blank | 19 |
| APTES | 72 |
| DHP acid-1 | 71 |
| DHP acid-2 | 73 |
| DHP acid-3 | 74 |
| DHP acid-4 | 75 |
| p-acid DHP | 68 |
| Compound | QS Inhibition against PA MH602 (%) | ||
|---|---|---|---|
| 62.5 µM | 125 µM | 250 µM | |
| DHP acid-1 | 27.6 ± 1.7a | 40.4 ± 1.2a | 56.3 ± 0.4b |
| DHP acid-2 | 33.5 ± 1.2a | 34.2 ± 2.3a | 55.3 ± 3.8a |
| DHP acid-3 | 33.0 ± 1.8a | 35.2 ± 2.0a | 57.5 ± 0.7b |
| DHP acid-4 | 31.0 ± 1.7a | 35.1 ± 1.1a | 53.6 ± 1.5a |
| p-acid DHP | 35.0 ± 2.4a | 38.5 ± 1.1b | 68.6 ± 1.1b |
| Furanone-30 (positive control) | 41.4 ± 2.7a | 64.2 ± 3.5b | 89.7 ± 4.6c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taunk, A.; Chen, R.; Iskander, G.; Ho, K.K.K.; Almohaywi, B.; Black, D.S.; Willcox, M.D.P.; Kumar, N. The Role of Orientation of Surface Bound Dihydropyrrol-2-ones (DHP) on Biological Activity. Molecules 2019, 24, 2676. https://doi.org/10.3390/molecules24142676
Taunk A, Chen R, Iskander G, Ho KKK, Almohaywi B, Black DS, Willcox MDP, Kumar N. The Role of Orientation of Surface Bound Dihydropyrrol-2-ones (DHP) on Biological Activity. Molecules. 2019; 24(14):2676. https://doi.org/10.3390/molecules24142676
Chicago/Turabian StyleTaunk, Aditi, Renxun Chen, George Iskander, Kitty K. K. Ho, Basmah Almohaywi, David StClair Black, Mark D. P. Willcox, and Naresh Kumar. 2019. "The Role of Orientation of Surface Bound Dihydropyrrol-2-ones (DHP) on Biological Activity" Molecules 24, no. 14: 2676. https://doi.org/10.3390/molecules24142676







