Synthesis of 6,12-Disubstituted Methanodibenzo[b,f][1,5]dioxocins: Pyrrolidine Catalyzed Self-Condensation of 2′-Hydroxyacetophenones
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. General Procedure for the Synthesis of Substituted Methanodibenzo[b,f][1,5]Dioxocin Derivatives 1–7
3.3. Single Crystal X-Ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Ibrar, A.; Shehzadi, S.A. Building molecular complexity through transition-metal-catalyzed oxidative annulations/cyclizations: Harnessing the utility of phenols, naphthols and 1,3-dicarbonyl compounds. Coord. Chem. Rev. 2019, 380, 440–470. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M. Organic chemistry: Synthetic lessons from nature. Nature 2009, 459, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M.L.; Sorensen, E.J. Rapid complexity generation in natural product total synthesis. Chem. Soc. Rev. 2009, 38, 2981–2982. [Google Scholar] [CrossRef]
- Hamann, L.G. Synthetic strategy: Natural products on demand. Nat. Chem. 2014, 6, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.B.; Simmons, B.; Mastracchio, A.; MacMillan, D.W. Collective synthesis of natural products by means of organocascade catalysis. Nature 2011, 475, 183–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellissier, H. Stereocontrolled domino reactions. Chem. Rev. 2013, 113, 442–524. [Google Scholar] [CrossRef]
- Razzak, M.; De Brabander, J.K. Lessons and revelations from biomimetic syntheses. Nat. Chem. Biol. 2011, 7, 865–875. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Econoomy-A search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Anderson, E.A. Cascade polycyclisations in natural product synthesis. Org. Biomol. Chem. 2011, 9, 3997–4006. [Google Scholar] [CrossRef]
- Ardkhean, R.; Caputo, D.F.; Morrow, S.M.; Shi, H.; Xiong, Y.; Anderson, E.A. Cascade polycyclizations in natural product synthesis. Chem. Soc. Rev. 2016, 45, 1557–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Movassaghi, M. Biogenetically inspired syntheses of alkaloid natural products. Chem. Soc. Rev. 2009, 38, 3035–3050. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Nay, B. Transition metal-promoted biomimetic steps in total syntheses. Nat. Prod. Res. 2014, 31, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Chen, J.S. The art of total synthesis through cascade reactions. Chem. Soc. Rev. 2009, 38, 2993–3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. Engl. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Lam, H.C.; Sumby, C.J.; George, J.H. Biomimetic Total Synthesis of Rhodonoids C and D, and Murrayakonine D. Org. Lett. 2017, 19, 2463–2465. [Google Scholar] [CrossRef]
- Han, Y.X.; Jiang, Y.L.; Li, Y.; Yu, H.X.; Tong, B.Q.; Niu, Z.; Zhou, S.J.; Liu, S.; Lan, Y.; Chen, J.H.; et al. Biomimetically inspired asymmetric total synthesis of (+)-19-dehydroxyl arisandilactone A. Nat. Commun. 2017, 8, 14233. [Google Scholar] [CrossRef] [Green Version]
- Kotammagari, T.K.; Gonnade, R.G.; Bhattacharya, A.K. Biomimetic Total Synthesis of Angiopterlactone B and Other Potential Natural Products. Org. Lett. 2017, 19, 3564–3567. [Google Scholar] [CrossRef]
- Lachkar, D.; Denizot, N.; Bernadat, G.; Ahamada, K.; Beniddir, M.A.; Dumontet, V.; Gallard, J.F.; Guillot, R.; Leblanc, K.; N’Nang, E.; et al. Unified biomimetic assembly of voacalgine A and bipleiophylline via divergent oxidative couplings. Nat. Chem. 2017, 9, 793–798. [Google Scholar] [CrossRef]
- Newton, C.G.; Tran, D.N.; Wodrich, M.D.; Cramer, N. One-Step Multigram-Scale Biomimetic Synthesis of Psiguadial B. Angew. Chem. Int. Ed. Engl. 2017, 56, 13776–13780. [Google Scholar] [CrossRef] [PubMed]
- Reichl, K.D.; Smith, M.J.; Song, M.K.; Johnson, R.P.; Porco, J.A., Jr. Biomimetic Total Synthesis of (+/−)-Griffipavixanthone via a Cationic Cycloaddition-Cyclization Cascade. J. Am. Chem. Soc. 2017, 139, 14053–14056. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Huang, F.; Guo, L.; Ouyang, M.A.; Tong, R. A cascade Claisen rearrangement/o-quinone methide formation/electrocyclization approach to 2H-chromenes. Chem. Commun. 2017, 53, 6021–6024. [Google Scholar] [CrossRef] [PubMed]
- Bam, M.; Ferreira, D.; Brandt, E.V. Novel cyanomaclurin analogue from Peltophorum africanum. Phytochemistry 1988, 27, 3704–3705. [Google Scholar] [CrossRef]
- Bhatia, G.D.; Mukerjee, S.K.; Seshadri, T.R. Synthesis of (±) trimethyl cyanomaclurin and its acetate. Tetrahedron 1966, 22, 531–540. [Google Scholar] [CrossRef]
- Cui, Y.M.; Wang, H.; Liu, Q.R.; Han, M.; Lu, Y.; Zhao, C.Q. Flavans from Iris tenuifolia and their effects on beta-amyloid aggregation and neural stem cells proliferation in vitro. Bioorg. Med. Chem. Lett. 2011, 21, 4400–4403. [Google Scholar] [CrossRef]
- Eastmond, R.; Gardner, R.J. [14C]epicatechin and [14C]procyanidins from seed shells of Aesculus hippocastanum. Phytochemistry 1974, 13, 1477–1478. [Google Scholar] [CrossRef]
- Lin, C.-N.; Lu, C.-M.; Huang, P.-L. Flavonoids from Artocarpus heterophyllus. Phytochemistry 1995, 39, 1447–1451. [Google Scholar] [CrossRef]
- Nair, P.M.; Venkataraman, K. Cyanomaclurin. Tetrahedron Lett. 1963, 4, 317–320. [Google Scholar] [CrossRef]
- Rashid, M.A.; Armstrong, J.A.; Gray, A.I.; Waterman, P.G. Tetra− and pentacyclic 6-c-monoterpenyl-5,7-dioxycoumarins from Eriostemon brucei and e. Brucei subspecies cinereus. Phytochemistry 1992, 31, 3583–3588. [Google Scholar] [CrossRef]
- Sasaki, H.; Kashiwada, Y.; Shibata, H.; Takaishi, Y. Prenylated flavonoids from Desmodium caudatum and evaluation of their anti-MRSA activity. Phytochemistry 2012, 82, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G.; Mahmoud, E.-H.N. Unusual flavonoids from Lonchocarpus orotinus seeds. Phytochemistry 1987, 26, 1189–1193. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Jing, Y.; Wang, G.C.; Wang, Y.; Zhao, H.N.; Ye, W.C. Four new flavonoids from the leaves of Morus mongolica. Fitoterapia 2010, 81, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.; Goll, L.; Arndt, E.M.V.; Mannschreck, A. Procyanidino-(-)-epicatechin, ein zweiarmig verknüpftes, kondensiertes proanthocyanidin aus aesculus hippocastanum. Tetrahedron Lett. 1966, 7, 429–435. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, X.; Liu, X.; Qian, C.; Che, X.; Cao, F.; Jin, S.; Meng, D. Caudatan A, an undescribed human kidney-type glutaminase inhibitor with tetracyclic flavan from Ohwia caudata. Phytochemistry 2018, 152, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Perkin, A.G.; Cope, F. XCV—The constituents of Artocarpus integrifolia. Part I. J. Chem. Soc. Trans. 1895, 67, 937–944. [Google Scholar] [CrossRef]
- Hennis, H.E.; Thompson, L.R.; Wang, C.-S.; Priddy, D.B. 6H,12H-6,12-Methanodibenzo[b,f][1,5]dioxocines from the reactions of o-alkenylphenols and salicylaldehydes. J. Org. Chem. 1970, 35, 3127–3129. [Google Scholar] [CrossRef]
- Hennis, H.E.; Wang, C.-S. 6H,12H-6,12-methanodibenzo[b,f] [1,5]dioxocins from the reactions of o-coumaric acids and salicylaldehydes. J. Org. Chem. 1969, 34, 1907–1911. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Guo, X.; Huo, L.; Xu, Z.; Zhang, W.; Qiu, S.; Yang, B.; Tan, H. A Bioinspired Cascade Sequence Enables Facile Assembly of Methanodibenzo[b,f][1,5]dioxocin Flavonoid Scaffold. Org. Lett. 2018, 20, 546–549. [Google Scholar] [CrossRef]
- Du, J.Y.; Ma, Y.H.; Meng, F.X.; Chen, B.L.; Zhang, S.L.; Li, Q.L.; Gong, S.W.; Wang, D.Q.; Ma, C.L. Lewis Acid Catalyzed Tandem 1,4-Conjugate Addition/Cyclization of in Situ Generated Alkynyl o-Quinone Methides and Electron-Rich Phenols: Synthesis of Dioxabicyclo [3.3.1]nonane Skeletons. Org. Lett. 2018, 20, 4371–4374. [Google Scholar] [CrossRef]
- Assoah, B.; Veiros, L.F.; Candeias, N.R. Pinacol-Derived Chlorohydrosilane in Metal-Free Reductive Amination for the Preparation of Tertiary Alkylphenolmethyl Amines. Org. Lett. 2019, 21, 1402–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assoah, B.; Vale, J.R.; Kalenius, E.; Veiros, L.F.; Candeias, N.R. Lewis Base Catalyzed Intramolecular Reduction of Salicylaldehydes by Pinacol-Derived Chlorohydrosilane. Eur. J. Org. Chem. 2018, 2018, 2910–2917. [Google Scholar] [CrossRef]
- Ragot, J.P.; Prime, M.E.; Archibald, S.J.; Taylor, R.J.K. A Novel Route to Preussomerins via 2-Arylacetal Anions. Org. Lett. 2000, 2, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.S.; Hosangadi, B.D. A Facile Synthesis of Anhydro-dimers of o-Hydroxybenzaldehydes. Synth. Commun. 1986, 16, 191–193. [Google Scholar] [CrossRef]
- Chen, J.R.; Yang, D.Y. Design and synthesis of an o-hydroxyphenyl-containing spiropyran thermochromic colorant. Org. Lett. 2009, 11, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Husch, T.; Seebach, D.; Beck, A.K.; Reiher, M. Rigorous Conformational Analysis of Pyrrolidine Enamines with Relevance to Organocatalysis. Helv. Chim. Acta 2017, 100, e1700182. [Google Scholar] [CrossRef] [Green Version]
- Kempf, B.; Hampel, N.; Ofial, A.R.; Mayr, H. Structure-nucleophilicity relationships for enamines. Chem. Eur. J. 2003, 9, 2209–2218. [Google Scholar] [CrossRef]
- Deb, M.L.; Dey, S.S.; Bento, I.; Barros, M.T.; Maycock, C.D. Copper-catalyzed regioselective intramolecular oxidative alpha-functionalization of tertiary amines: An efficient synthesis of dihydro-1,3-oxazines. Angew. Chem. Int. Ed. Engl. 2013, 52, 9791–9795. [Google Scholar] [CrossRef]
- Kabbe, H.-J.; Widdig, A. Synthesen und Umsetzungen von 4-Chromanonen. Angew. Chem. 1982, 94, 254–262. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.38.43; Rigaku Oxford Diffraction: Yarnton, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |



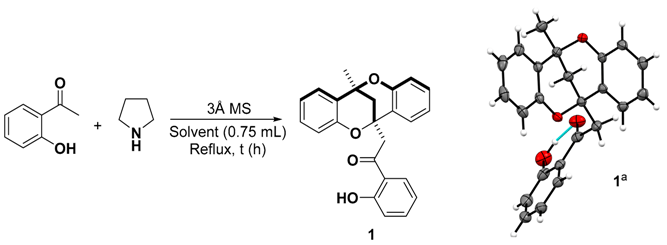
| Entry b | Pyrrolidine (equiv.) | Solvent | Conditions | Time (h) | Yield (%) c |
|---|---|---|---|---|---|
| 1 | 1 | CH3CN | Reflux d | 18 | 24 |
| 2 | 1 | CH3CN | Reflux d, no MS | 18 | n.d. e |
| 3 | none | CH3CN | Reflux d | 18 | n.d. e |
| 4 | none | CH3CN | dry p-TSA (0.2 equiv) d | 48 | n.d. e |
| 5 | - | Pyrrolidine g | Reflux | 21 | n.d. e |
| 6 | 0.3 | CH3CN | Reflux | 24 | 42 |
| 7 | 0.3 | none | Sealed tube | 24 | 41 (59) f |
| 8 | 0.15 | none | Sealed tube | 24 | 29 |
| 9 | 0.15 | CH3CN | Sealed tube | 24 | 36 |
| 10 | 0.05 | CH3CN | Sealed tube | 24 | 25 |
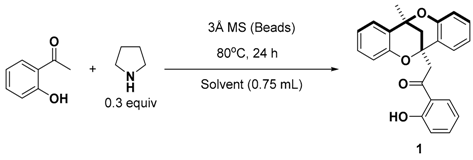
| Entry | Solvent | Isolated Yield (%) | |
|---|---|---|---|
| Sealed Tube | Open Vessel | ||
| 1 | CH3CN | 29 | 42 |
| 2 | EtOH | n.d. a | - |
| 3 | DMSO | 28 | - |
| 4 | MTBE | 32 | 43 |
| 5 | 1,4-Dioxane | - | 30 |
| 6 | DCE | trace | 15 |
| 7 | Hexane | 44 b (39) c | 53 (50) d |
| 8 | Toluene | 31 e | 27 |
| 9 | Heptane | - | 45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assoah, B.; Riihonen, V.; Vale, J.R.; Valkonen, A.; Candeias, N.R. Synthesis of 6,12-Disubstituted Methanodibenzo[b,f][1,5]dioxocins: Pyrrolidine Catalyzed Self-Condensation of 2′-Hydroxyacetophenones. Molecules 2019, 24, 2405. https://doi.org/10.3390/molecules24132405
Assoah B, Riihonen V, Vale JR, Valkonen A, Candeias NR. Synthesis of 6,12-Disubstituted Methanodibenzo[b,f][1,5]dioxocins: Pyrrolidine Catalyzed Self-Condensation of 2′-Hydroxyacetophenones. Molecules. 2019; 24(13):2405. https://doi.org/10.3390/molecules24132405
Chicago/Turabian StyleAssoah, Benedicta, Vesa Riihonen, João R. Vale, Arto Valkonen, and Nuno R. Candeias. 2019. "Synthesis of 6,12-Disubstituted Methanodibenzo[b,f][1,5]dioxocins: Pyrrolidine Catalyzed Self-Condensation of 2′-Hydroxyacetophenones" Molecules 24, no. 13: 2405. https://doi.org/10.3390/molecules24132405
APA StyleAssoah, B., Riihonen, V., Vale, J. R., Valkonen, A., & Candeias, N. R. (2019). Synthesis of 6,12-Disubstituted Methanodibenzo[b,f][1,5]dioxocins: Pyrrolidine Catalyzed Self-Condensation of 2′-Hydroxyacetophenones. Molecules, 24(13), 2405. https://doi.org/10.3390/molecules24132405






