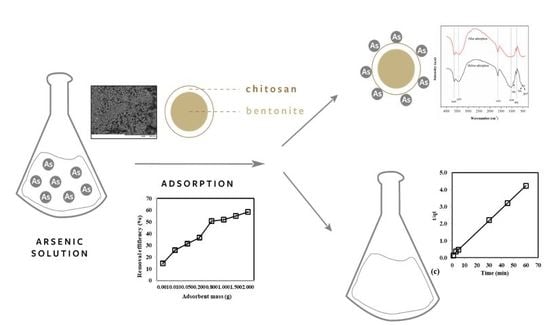
Treatment of Contaminated Groundwater via Arsenate Removal Using Chitosan-Coated Bentonite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of CCB
2.4. Adsorption Experiments
2.5. Error Analysis
3. Results and Discussion
3.1. Characterization
3.2. Effect of Adsorbent Mass, Contact Time, and Temperature
3.3. Kinetic Study
3.4. Isotherm Study
3.5. Thermodynamics Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DeSesso, J.M.; Jacobson, C.F.; Scialli, A.R.; Farr, C.H.; Holson, J.F. An assessment of the developmental toxicity of inorganic arsenic. Reprod. Toxicol. 1998, 12, 385–433. [Google Scholar] [CrossRef]
- Chakravarty, S.; Dureja, V.; Bhattacharyya, G.; Maity, S.; Bhattacharjee, S. Removal of arsenic from groundwater using low cost ferruginous manganese ore. Water Res. 2002, 36, 625–632. [Google Scholar] [CrossRef]
- Camacho, L.M.; Gutiérrez, M.; Alarcón-Herrera, M.T.; Villalba, M.L.; Deng, S. Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere 2011, 83, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Hafeznezami, S.; Zimmer-Faust, A.G.; Jun, D.; Rugh, M.B.; Haro, H.L.; Park, A.; Suh, J.; Najm, T.; Reynolds, M.D.; Davis, J.A.; et al. Remediation of groundwater contaminated with arsenic through enhanced natural attenuation: Batch and column studies. Water Res. 2017, 122, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; De, S. Adsorptive removal of arsenic from groundwater using chemically treated iron ore slime incorporated mixed matrix hollow fiber membrane. Sep. Purif. Technol. 2017, 179, 357–368. [Google Scholar] [CrossRef]
- Farooq, S.H.; Chandrasekharam, D.; Berner, Z.; Norra, S.; Stüben, D. Influence of traditional agricultural practices on mobilization of arsenic from sediments to groundwater in Bengal delta. Water Res. 2010, 44, 5575–5588. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Hosono, T.; Siringan, F.; Yamanaka, T.; Umezawa, Y.; Onodera, S.; Nakano, T.; Taniguchi, M. Application of multi-isotope ratios to study the source and quality of urban groundwater in Metro Manila, Philippines. Appl. Geochem. 2010, 25, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Maji, S.K.; Kao, Y.-H.; Liu, C.-W. Arsenic removal from real arsenic-bearing groundwater by adsorption on iron-oxide-coated natural rock (IOCNR). Desalination 2011, 280, 72–79. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pontius, F.W. Crafting a New Arsenic Rule. J. Am. Water Works Assoc. 1994, 86, 6–104. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Guibal, E. Arsenic(V) sorption using chitosan/Cu(OH)2 and chitosan/CuO composite sorbents. Carbohydr. Polym. 2015, 134, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.; Styblo, M.; Lin, S. The Cellular Metabolism and Systemic Toxicity of Arsenic. Toxicol. Appl. Pharmacol. 2001, 176, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamauchi, H.; Fan Sun, G. Chronic health effects in people exposed to arsenic via the drinking water: Dose–response relationships in review. Toxicol. Appl. Pharmacol. 2004, 198, 243–252. [Google Scholar] [CrossRef]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chung, Y.-C. Arsenic removal using a biopolymer chitosan sorbent. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2006, 41, 645–658. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, G.; Li, H. Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresour. Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef]
- Shekhawat, A.; Kahu, S.; Saravanan, D.; Jugade, R. Tin(IV) cross-linked chitosan for the removal of As(III). Carbohydr. Polym. 2017, 172, 205–212. [Google Scholar] [CrossRef]
- Roghani, M.; Nakhli, S.A.A.; Aghajani, M.; Rostami, M.H.; Borghei, S.M. Adsorption and oxidation study on arsenite removal from aqueous solutions by polyaniline/polyvinyl alcohol composite. J. Water Process Eng. 2016, 14, 101–107. [Google Scholar] [CrossRef]
- Uppal, H.; Hemlata; Tawale, J.; Singh, N. Zinc peroxide functionalized synthetic graphite: An economical and efficient adsorbent for adsorption of arsenic (III) and (V). J. Environ. Chem. Eng. 2016, 4, 2964–2975. [Google Scholar] [CrossRef]
- Martin, T.A.; Kempton, J.H. In Situ Stabilization of Metal-Contaminated Groundwater by Hydrous Ferric Oxide: An Experimental and Modeling Investigation. Environ. Sci. Technol. 2000, 34, 3229–3234. [Google Scholar] [CrossRef]
- Statham, T.M.; Stark, S.C.; Snape, I.; Stevens, G.W.; Mumford, K.A. A permeable reactive barrier (PRB) media sequence for the remediation of heavy metal and hydrocarbon contaminated water: A field assessment at Casey Station, Antarctica. Chemosphere 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Day, S.R.; O’Hannesin, S.F.; Marsden, L. Geotechnical techniques for the construction of reactive barriers. J. Hazard. Mater. 1999, 67, 285–297. [Google Scholar] [CrossRef]
- Trace Metals and Other Contaminants in the Environment. Long-Term Performance of Permeable Reactive Barriers. ScienceDirect.com. Available online: https://www.sciencedirect.com/bookseries/trace-metals-and-other-contaminants-in-the-environment/vol/7 (accessed on 1 June 2019).
- Saha, S.; Sarkar, P. Arsenic remediation from drinking water by synthesized nano-alumina dispersed in chitosan-grafted polyacrylamide. J. Hazard. Mater. 2012, 227–228, 68–78. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel Metal–Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th(IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef]
- Gogoi, P.; Thakur, A.J.; Devi, R.R.; Das, B.; Maji, T.K. A comparative study on sorption of arsenate ions from water by crosslinked chitosan and crosslinked chitosan/MMT nanocomposite. J. Environ. Chem. Eng. 2016, 4, 4248–4257. [Google Scholar] [CrossRef]
- Arida, C.V.J.; de Luna, M.D.G.; Futalan, C.M.; Wan, M.-W. Optimization of As(V) removal using chitosan-coated bentonite from groundwater using Box–Behnken design: Effects of adsorbent mass, flow rate, and initial concentration. Desalin. Water Treat. 2016, 57, 18739–18747. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Mattusch, J.; Wennrich, R.; Morgenstern, P. Uptake of arsenite and arsenate by clinoptilolite-rich tuffs. Microporous Mesoporous Mater. 2001, 46, 277–286. [Google Scholar] [CrossRef]
- Soner Altundoğan, H.; Altundoğan, S.; Tümen, F.; Bildik, M. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, D.; Chaudhury, G.R.; Das, R.P. Arsenic(V) adsorption mechanism using kaolinite, montmorillonite and illite from aqueous medium. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2007, 42, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Parette, R.; Zou, J.; Cannon, F.S.; Dempsey, B.A. Arsenic removal by iron-modified activated carbon. Water Res. 2007, 41, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Suazo-Hernández, J.; Sepúlveda, P.; Manquián-Cerda, K.; Ramírez-Tagle, R.; Rubio, M.A.; Bolan, N.; Sarkar, B.; Arancibia-Miranda, N. Synthesis and characterization of zeolite-based composites functionalized with nanoscale zero-valent iron for removing arsenic in the presence of selenium from water. J. Hazard. Mater. 2019, 373, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Vitela-Rodriguez, A.V.; Rangel-Mendez, J.R. Arsenic removal by modified activated carbons with iron hydro(oxide) nanoparticles. J. Environ. Manag. 2013, 114, 225–231. [Google Scholar] [CrossRef]
- Urbano, B.F.; Rivas, B.L.; Martinez, F.; Alexandratos, S.D. Water-insoluble polymer–clay nanocomposite ion exchange resin based on N-methyl-d-glucamine ligand groups for arsenic removal. React. Funct. Polym. 2012, 72, 642–649. [Google Scholar] [CrossRef]
- Gupta, K.; Ghosh, U.C. Arsenic removal using hydrous nanostructure iron(III)-titanium(IV) binary mixed oxide from aqueous solution. J. Hazard. Mater. 2009, 161, 884–892. [Google Scholar] [CrossRef]
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II), and Ni(II) From Single- and Multimetal Solutions by Recycled Waste Porous Glass. Chem. Eng. Commun. 2016, 203, 940–947. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Rizzi, V.; Cosma, P.; Race, M.; De Vietro, N. Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field. Appl. Sci. 2018, 8, 1882. [Google Scholar] [CrossRef]
- Kausar, A.; Naeem, K.; Hussain, T.; Nazli, Z.-H.; Bhatti, H.N.; Jubeen, F.; Nazir, A.; Iqbal, M. Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: Comparison of linear and non-linear regression methods. J. Mater. Res. Technol. 2019, 8, 1161–1174. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption behaviors of Congo red on the N,O-carboxymethyl-chitosan/montmorillonite nanocomposite. Chem. Eng. J. 2008, 143, 43–50. [Google Scholar] [CrossRef]
- Brion-Roby, R.; Gagnon, J.; Deschênes, J.-S.; Chabot, B. Investigation of fixed bed adsorption column operation parameters using a chitosan material for treatment of arsenate contaminated water. J. Environ. Chem. Eng. 2018, 6, 505–511. [Google Scholar] [CrossRef]
- Lalhmunsiama; Lalchhingpuii; Nautiyal, B.P.; Tiwari, D.; Choi, S.I.; Kong, S.-H.; Lee, S.-M. Silane grafted chitosan for the efficient remediation of aquatic environment contaminated with arsenic(V). J. Colloid Interface Sci. 2016, 467, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Helios-Rybicka, E.; Wójcik, R. Competitive sorption/desorption of Zn, Cd, Pb, Ni, Cu, and Cr by clay-bearing mining wastes. Appl. Clay Sci. 2012, 65–66, 6–13. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010, 160, 157–163. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-W.; Jeon, B.-H.; Chon, C.-M.; Kim, Y.; Schwartz, F.W.; Lee, E.-S.; Song, H. A novel chitosan/clay/magnetite composite for adsorption of Cu(II) and As(V). Chem. Eng. J. 2012, 200–202, 654–662. [Google Scholar] [CrossRef]
- Futalan, C.M.; Huang, Y.-S.; Chen, J.-H.; Wan, M.-W. Arsenate removal from aqueous solution using chitosan-coated bentonite, chitosan-coated kaolinite and chitosan-coated sand: Parametric, isotherm and thermodynamic studies. Water Sci. Technol. 2018, 78, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.-W.; Kan, C.-C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Ho, Y.-S. Removal of lead (II) ions from synthetic and real effluents using immobilized Pinus sylvestris sawdust: Adsorption on a fixed-bed column. J. Hazard. Mater. 2005, 123, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Maliyekkal, S.M.; Shukla, S.; Philip, L.; Nambi, I.M. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules. Chem. Eng. J. 2008, 140, 183–192. [Google Scholar] [CrossRef]
- Gerard, N.; Santhana Krishnan, R.; Ponnusamy, S.K.; Cabana, H.; Vaidyanathan, V.K. Adsorptive potential of dispersible chitosan coated iron-oxide nanocomposites toward the elimination of arsenic from aqueous solution. Process Saf. Environ. Prot. 2016, 104, 185–195. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Devi, N.; Dutta, J. Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int. J. Biol. Macromol. 2017, 104, 1897–1904. [Google Scholar] [CrossRef]
- Liu, Z.; Azhar Uddin, M.; Sun, Z. FT-IR and XRD analysis of natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar]
- Zavareh, S.; Zarei, M.; Darvishi, F.; Azizi, H. As(III) adsorption and antimicrobial properties of Cu–chitosan/alumina nanocomposite. Chem. Eng. J. 2015, 273, 610–621. [Google Scholar] [CrossRef]
- Saini, A.S.; Melo, J.S. Biosorption of uranium by melanin: Kinetic, equilibrium and thermodynamic studies. Bioresour. Technol. 2013, 149, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-T.; Saman, N.; Johari, K.; Mat, H. Removal of Hg(II) from Aqueous Solution by Adsorption Using Raw and Chemically Modified Rice Straw As Novel Adsorbents. Ind. Eng. Chem. Res. 2013, 52, 13092–13101. [Google Scholar] [CrossRef]
- Benkaddour, S.; Slimani, R.; Hiyane, H.; El Ouahabi, I.; Hachoumi, I.; El Antri, S.; Lazar, S. Removal of reactive yellow 145 by adsorption onto treated watermelon seeds: Kinetic and isotherm studies. Sustain. Chem. Pharm. 2018, 10, 16–21. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristics curve of activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Delle Site, A. Factors Affecting Sorption of Organic Compounds in Natural Sorbent/Water Systems and Sorption Coefficients for Selected Pollutants. A Review. J. Phys. Chem. Ref. Data 2001, 30, 187–439. [Google Scholar] [CrossRef] [Green Version]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.A.; González, J.C.; García, S.I.; Sala, L.F.; Bellú, S.E. Application of chitosan in removal of molybdate ions from contaminated water and groundwater. Carbohydr. Polym. 2018, 180, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Calagui, M.J.C.; Senoro, D.B.; Kan, C.-C.; Salvacion, J.W.L.; Futalan, C.M.; Wan, M.-W. Adsorption of indium(III) ions from aqueous solution using chitosan-coated bentonite beads. J. Hazard. Mater. 2014, 277, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Khatamian, M.; Khodakarampoor, N.; Saket-Oskoui, M. Efficient removal of arsenic using graphene-zeolite based composites. J. Colloid Interface Sci. 2017, 498, 433–441. [Google Scholar] [CrossRef]
- Arcibar-Orozco, J.A.; Josue, D.-B.; Rios-Hurtado, J.C.; Rangel-Mendez, J.R. Influence of iron content, surface area and charge distribution in the arsenic removal by activated carbons. Chem. Eng. J. 2014, 249, 201–209. [Google Scholar] [CrossRef]
- Barnie, S.; Zhang, J.; Wang, H.; Yin, H.; Chen, H. The influence of pH, co-existing ions, ionic strength, and temperature on the adsorption and reduction of hexavalent chromium by undissolved humic acid. Chemosphere 2018, 212, 209–218. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M.; Grimes, R. Advanced Inorganic Chemistry, 6th ed.; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Feng, Q.; Li, X.; Cheng, Y.; Meng, L.; Meng, Q. Removal of Humic Acid from Groundwater by Electrocoagulation. J. China Univ. Min. Technol. 2007, 17, 513–520. [Google Scholar] [CrossRef]
- Kan, C.-C.; Aganon, M.C.; Futalan, C.M.; Dalida, M.L.P. Adsorption of Mn2+ from aqueous solution using Fe and Mn oxide-coated sand. J. Environ. Sci. 2013, 25, 1483–1491. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, E.A.; de Bussetti, S.G. Thermodynamic parameters of adsorption of 1,10-phenanthroline and 2,2′-bipyridyl on hematite, kaolinite and montmorillonites. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 117–128. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Abdullah Alqadami, A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]








| Parameters | Unit | Values | Parameters | Unit | Values |
|---|---|---|---|---|---|
| pH | 7.9 | As(V) | µg/L | 50.99 | |
| Conductivity | μS/cm | 84.3 | K+ | mg/L | 30.52 |
| Eh | mV | 131.2 | Ca2+ | mg/L | 22.66 |
| Dissolved oxygen | mg/L | 3.1 | Na+ | mg/L | 631.77 |
| Resistivity | kΩ | 11.2 | Fe2+ | mg/L | 0.64 |
| TOC | mg/L | 4.3 | Mg2+ | mg/L | 0.28 |
| Turbidity | NTU | 17.2 | Cl− | mg/L | 253.00 |
| COD | mg/L | 6.7 | SO42− | mg/L | 36.34 |
| TDS | mg/L | 52.3 | PO42− | mg/L | 67.17 |
| Kinetic Model | Parameter | Values |
|---|---|---|
| Pseudo-first order | k1 (min−1) | 0.0117 |
| qe,theo (µg/g) | 2.201 | |
| R2 | 0.9013 | |
| χ2 | 31.26 | |
| RMSE | 22.05 | |
| Pseudo-second order | k2 (g/µg•min) | 4.502 × 10−3 |
| qe,theo (µg/g) | 8.340 | |
| R2 | 0.9951 | |
| χ2 | 8.33 | |
| RMSE | 4.31 | |
| Intraparticle diffusion | kp (g/µg•min1/2) | 1.519 |
| C | 6.408 | |
| R2 | 0.8450 | |
| χ2 | 77.22 | |
| RMSE | 45.93 | |
| Film diffusion | kfd (min−1) | 0.0353 |
| R2 | 0.9256 | |
| χ2 | 77.22 | |
| RMSE | 45.93 |
| Isotherm | Parameters | Values |
|---|---|---|
| Langmuir | KL (L/mg) | 0.0019 |
| qm (mg/g) | 1.4660 | |
| RL | 11.6690 | |
| R2 | 0.9899 | |
| χ2 | 0.91 | |
| RMSE | 4.87 | |
| Freundlich | n (g/L) | 0.2142 |
| Kf (mg/g) | 0.1538 | |
| R2 | 0.6508 | |
| χ2 | 11.03 | |
| RMSE | 18.75 | |
| D-R | B (mol2/kJ2) | 0.6069 |
| qm (mg/g) | 0.2704 | |
| E (kJ/mole) | 0.9077 | |
| R2 | 0.6259 | |
| χ2 | 13.83 | |
| RMSE | 22.64 |
| Adsorbent | qe (mg/g) | Reference |
|---|---|---|
| F400 (granular activated carbon) | 1.01 | Vitela-Rodriguez and Rangel-Mendez, 2013 [38] |
| Chitosan/glutaraldehyde | 2.86 | Gogoi et al., 2016 [30] |
| Clay/chitosan/glutaraldehyde | 3.40 | Gogoi et al., 2016 [30] |
| UltraCarb (activated carbon) | 43.6 | Chen et al., 2007 [36] |
| Polymer-clay nanocomposite ion exchange resin | 55.0 | Urbano et al., 2012 [39] |
| nZVI-zeolite | 38.3 | Suazo-Hernández et al., 2019 [37] |
| CCB | 1.47 | Present study |
| Temperature (K) | Ea (J/mol) | ΔH0 (J/mol) | ΔS0 (kJ/mol•K) | ΔG0 (kJ/mol) |
|---|---|---|---|---|
| 298 | 14.72 | 8.31 | 29.10 | 3.89 |
| 308 | 3.32 | |||
| 318 | 3.31 | |||
| 328 | 2.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, J.-J.; Arida, C.V.J.; Futalan, C.M.; de Luna, M.D.G.; Wan, M.-W. Treatment of Contaminated Groundwater via Arsenate Removal Using Chitosan-Coated Bentonite. Molecules 2019, 24, 2464. https://doi.org/10.3390/molecules24132464
Yee J-J, Arida CVJ, Futalan CM, de Luna MDG, Wan M-W. Treatment of Contaminated Groundwater via Arsenate Removal Using Chitosan-Coated Bentonite. Molecules. 2019; 24(13):2464. https://doi.org/10.3390/molecules24132464
Chicago/Turabian StyleYee, Jurng-Jae, Carlo Vic Justo Arida, Cybelle Morales Futalan, Mark Daniel Garrido de Luna, and Meng-Wei Wan. 2019. "Treatment of Contaminated Groundwater via Arsenate Removal Using Chitosan-Coated Bentonite" Molecules 24, no. 13: 2464. https://doi.org/10.3390/molecules24132464