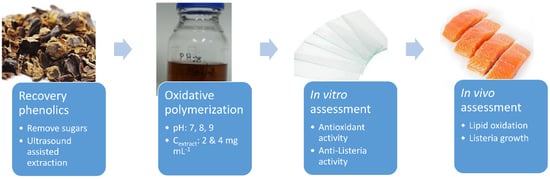
Valorization of Carob Fruit Residues for the Preparation of Novel Bi-Functional Polyphenolic Coating for Food Packaging Applications
Abstract
:1. Introduction
2. Results
2.1. Preparation of Coatings
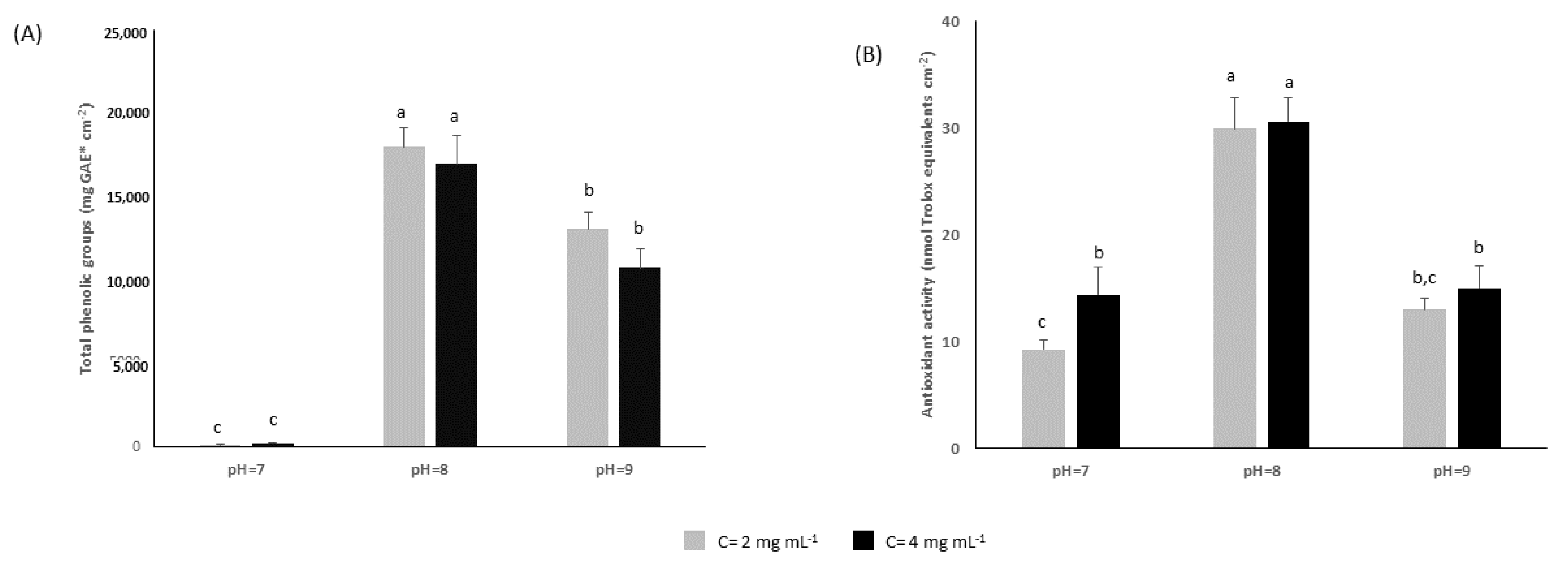
2.2. In Vitro Assessment of Polyphenolic Coatings
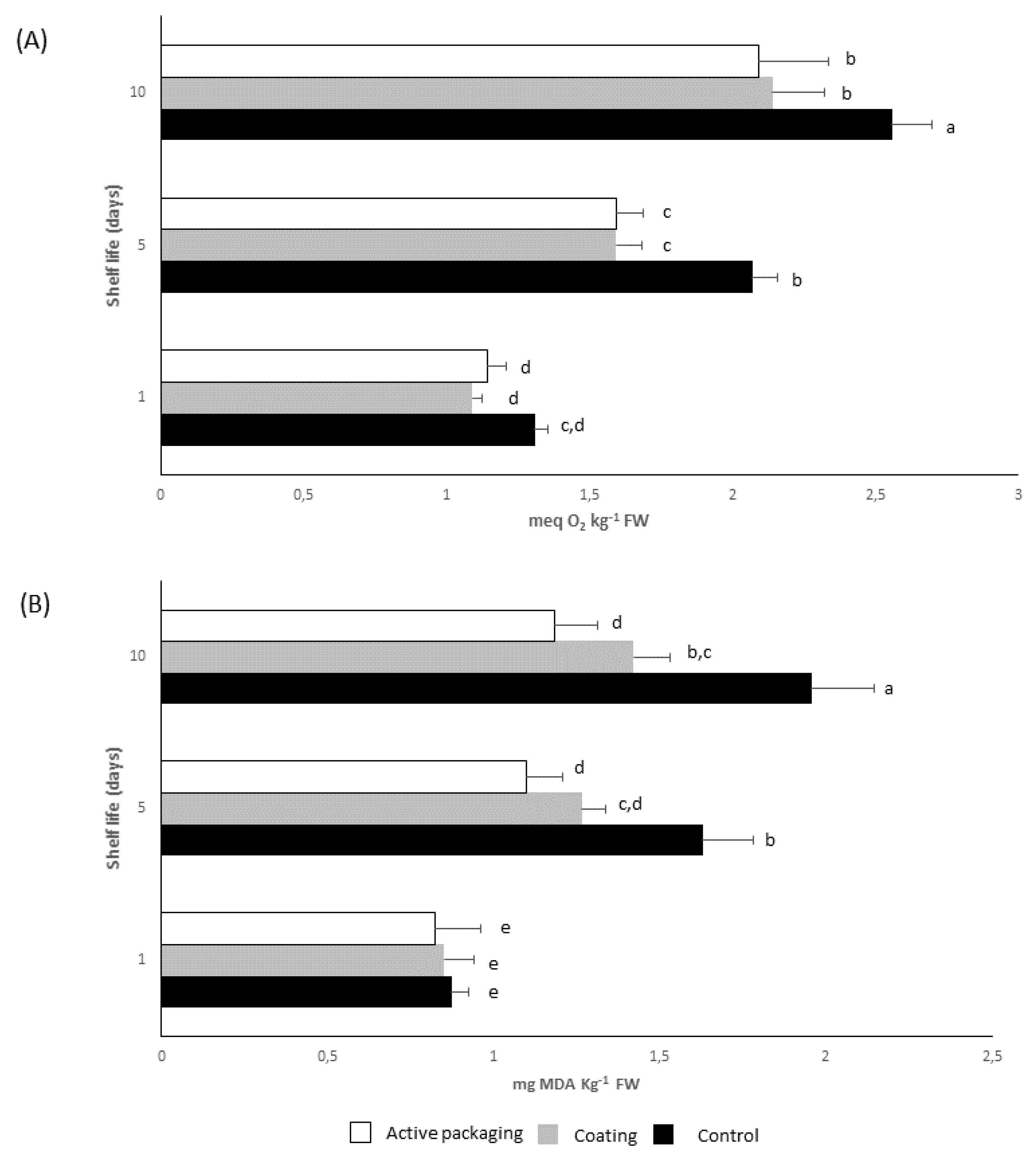
2.3. In Vivo Assessment of Polyphenolic Coatings
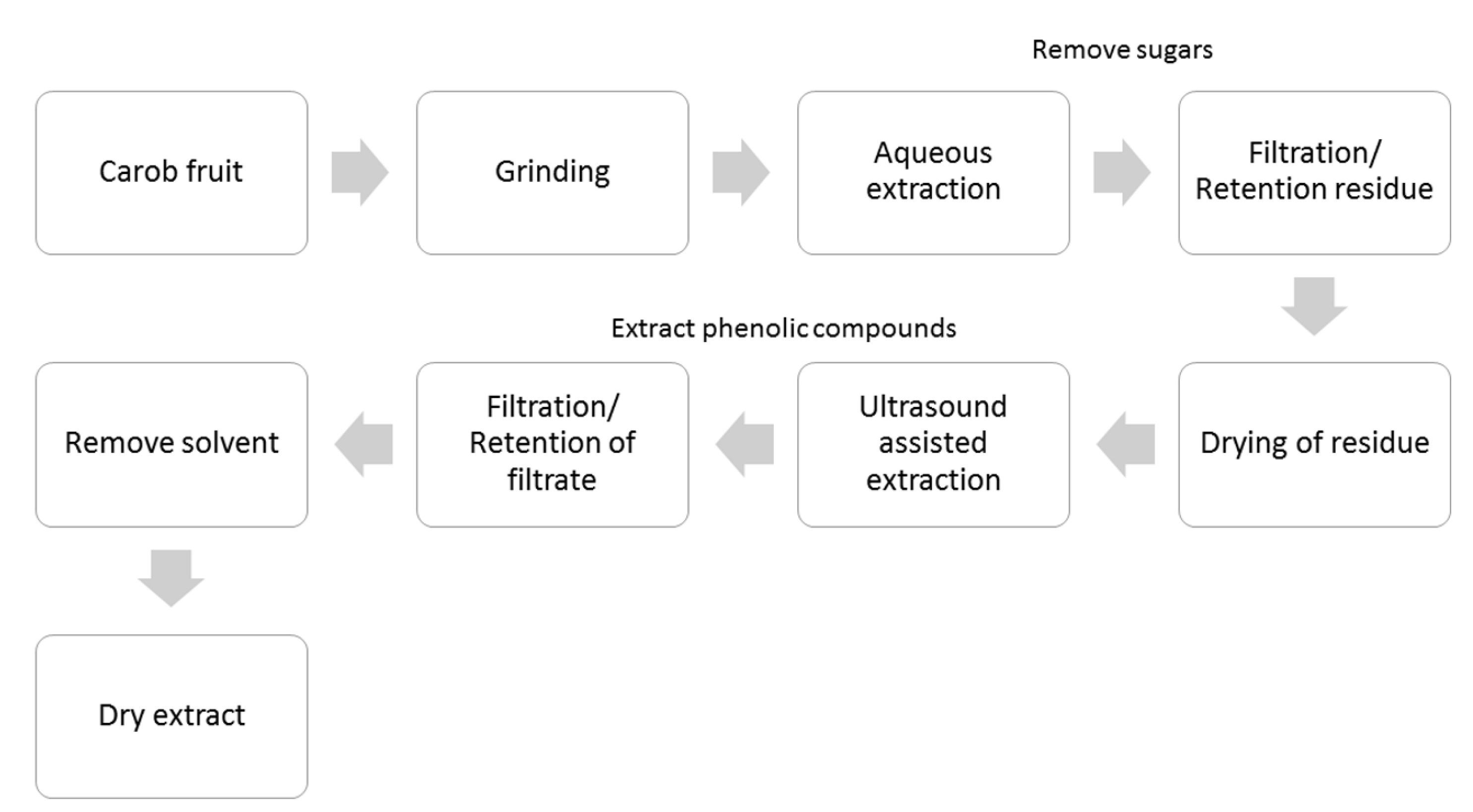
3. Materials and Methods
3.1. Chemicals and Carob Products
3.2. Preparation Coatings at Different pH Values and at Different Concentrations of Extract
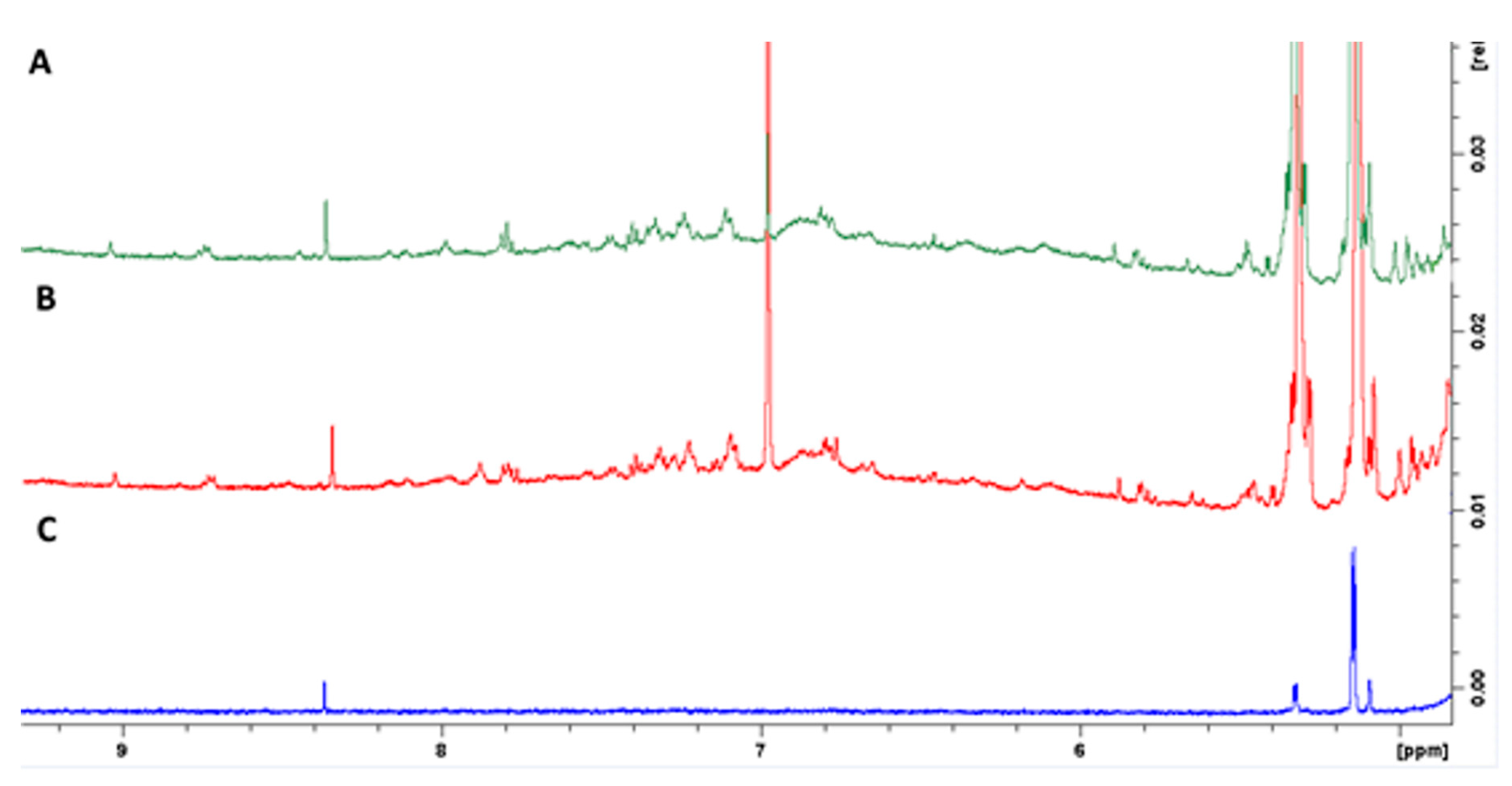
3.3. Screening of Carob Phenolic Polymerization by Nuclear Magnetic Resonance (NMR) Spectroscopy
3.4. Determination of Total Phenol Content and Antioxidant Potency of Coatings
3.5. Determination of Anti-Listeria Activity of Coatings
3.6. Application of Carob Polyphenolic Coating on the Salmon
3.7. Measurement the Inhibition of Lipid Oxidation by TBARS and Peroxide Value Assays
3.8. Measurement the Inhibition of Listeria Growth
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerrero, P.; Arana, P.; O’Grady, M.; Kerry, J.; De La Caba, K. Valorization of industrial by-products: development of active coatings to reduce food losses. J. Clean. Prod. 2015, 100, 179–184. [Google Scholar] [CrossRef]
- Yokokawa, N.; Kikuchi-Uehara, E.; Sugiyama, H.; Hirao, M. Framework for analyzing the effects of packaging on food loss reduction by considering consumer behavior. J. Clean. Prod. 2018, 174, 26–34. [Google Scholar] [CrossRef]
- Herbes, C.; Beuthner, C.; Ramme, I. Consumer attitudes towards biobased packaging—A cross-cultural comparative study. J. Clean. Prod. 2018, 194, 203–218. [Google Scholar] [CrossRef]
- Mkandawire, M.; Na Aryee, A.; Aryee, A. Resurfacing and modernization of edible packaging material technology. Curr. Opin. Food Sci. 2018, 19, 104–112. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Emerging trends in food packaging. Nutr. Food Sci. 2018, 48, 764–779. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, D.G.; Sileika, T.S.; Messersmith, P.B. Molecular diversity in phenolic and polyphenolic precursors of tannin-inspired nanocoatings. Chem. Commun. 2014, 50, 7265–7268. [Google Scholar] [CrossRef] [Green Version]
- Payra, D.; Naito, M.; Fujii, Y.; Nagao, Y. Hydrophobized plant polyphenols: self-assembly and promising antibacterial, adhesive, and anticorrosion coatings. Chem. Commun. 2016, 52, 312–315. [Google Scholar] [CrossRef]
- Xu, L.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T. Tea Stains-Inspired Antifouling Coatings Based on Tannic Acid-Functionalized Agarose. ACS Sustain. Chem. Eng. 2017, 5, 3055–3062. [Google Scholar] [CrossRef]
- Bai, G.; Ma, S.; Qie, R.; Liu, Z.; Shi, Y.; Li, C.; Wang, R.; Guo, X.; Zhou, F.; Jia, X. UV-Triggered Surface-Initiated Polymerization from Colorless Green Tea Polyphenol-Coated Surfaces. Macromol. Rapid Commun. 2016, 37, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Huang, J.; Zhang, S.; Wang, Y.; Gu, S.; Cao, G.; Yang, H.; Ye, D.; Zhou, Y.; Xu, W. Robust superhydrophobic surface by nature-inspired polyphenol chemistry for effective oil-water separation. Appl. Surf. Sci. 2018, 440, 535–546. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Yuan, X.; Wang, P.; Yang, J.; Miao, L. Highly transparent thermoresponsive surfaces based on tea-stain-inspired chemistry. J. Appl. Polym. Sci. 2018, 135, 46694. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Ejima, H.; Yoshie, N. Antioxidant and Adsorption Properties of Bioinspired Phenolic Polymers: A Comparative Study of Catechol and Gallol. ACS Sustain. Chem. Eng. 2016, 4, 3857–3863. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef]
- Roger, J.D.A.; Magro, M.; Spagnolo, S.; Bonaiuto, E.; Baratella, D.; Fasolato, L.; Vianello, F. Antimicrobial and magnetically removable tannic acid nanocarrier: A processing aid for Listeria monocytogenes treatment for food industry applications. Food Chem. 2018, 267, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Heu, M.S.; Park, C.H.; Lim, H.J.; Lee, D.H.; Kim, J.S. Effects of a gelatin coating on the self-life of salmon. Fish. Aqua Sci. 2010, 13, 89–95. [Google Scholar] [CrossRef]
- Botsaris, G.; Orphanides, A.; Yiannakou, E.; Gekas, V.; Goulas, V. Antioxidant and Antimicrobial Effects of Pistacia lentiscus L. Extracts in Pork Sausages. Food Technol. Biotechnol. 2015, 53, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 °C. Food Control 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Kim, I.-H.; Yang, H.-J.; Noh, B.-S.; Chung, S.-J.; Min, S.C. Development of a defatted mustard meal-based composite film and its application to smoked salmon to retard lipid oxidation. Food Chem. 2012, 133, 1501–1509. [Google Scholar] [CrossRef]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria monocytogenesin Aquatic Food Products-A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Retaining Oxidative Stability of Emulsified Foods by Novel Nonmigratory Polyphenol Coated Active Packaging. J. Agric. Food Chem. 2016, 64, 5574–5582. [Google Scholar] [CrossRef] [PubMed]
- Nithya, V.; Murthy, P.; Halami, P. Development and application of active films for food packaging using antibacterial peptide ofBacillus licheniformisMe1. J. Appl. Microbiol. 2013, 115, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Botsaris, G.; Taki, A. Effect of High-Pressure Processing on the microbial quality throughout the shelf life of vacuum-packed sliced ham and frankfurters. J. Food Process. Preserv. 2015, 39, 840–845. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Coating | Anti-Listeria Activity | Coated Slides |
|---|---|---|
| Control | − |  |
| pH 7 (2 mg mL−1) | + |  |
| pH 7 (4 mg mL−1) | + | |
| pH 8 (2 mg mL−1) | +++ |  |
| pH 8 (4 mg mL−1) | +++ | |
| pH 9 (2 mg mL−1) | ++ |  |
| pH 9 (4 mg mL−1) | ++ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulas, V.; Hadjivasileiou, L.; Primikyri, A.; Michael, C.; Botsaris, G.; Tzakos, A.G.; Gerothanassis, I.P. Valorization of Carob Fruit Residues for the Preparation of Novel Bi-Functional Polyphenolic Coating for Food Packaging Applications. Molecules 2019, 24, 3162. https://doi.org/10.3390/molecules24173162
Goulas V, Hadjivasileiou L, Primikyri A, Michael C, Botsaris G, Tzakos AG, Gerothanassis IP. Valorization of Carob Fruit Residues for the Preparation of Novel Bi-Functional Polyphenolic Coating for Food Packaging Applications. Molecules. 2019; 24(17):3162. https://doi.org/10.3390/molecules24173162
Chicago/Turabian StyleGoulas, Vlasios, Loukas Hadjivasileiou, Alexandra Primikyri, Christodoulos Michael, George Botsaris, Andreas G. Tzakos, and Ioannis P. Gerothanassis. 2019. "Valorization of Carob Fruit Residues for the Preparation of Novel Bi-Functional Polyphenolic Coating for Food Packaging Applications" Molecules 24, no. 17: 3162. https://doi.org/10.3390/molecules24173162
APA StyleGoulas, V., Hadjivasileiou, L., Primikyri, A., Michael, C., Botsaris, G., Tzakos, A. G., & Gerothanassis, I. P. (2019). Valorization of Carob Fruit Residues for the Preparation of Novel Bi-Functional Polyphenolic Coating for Food Packaging Applications. Molecules, 24(17), 3162. https://doi.org/10.3390/molecules24173162