Preclinical Study of DNA-Recognized Peptide Compound Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Progressive Renal Diseases in the Common Marmoset
Abstract
:1. Introduction
2. Results
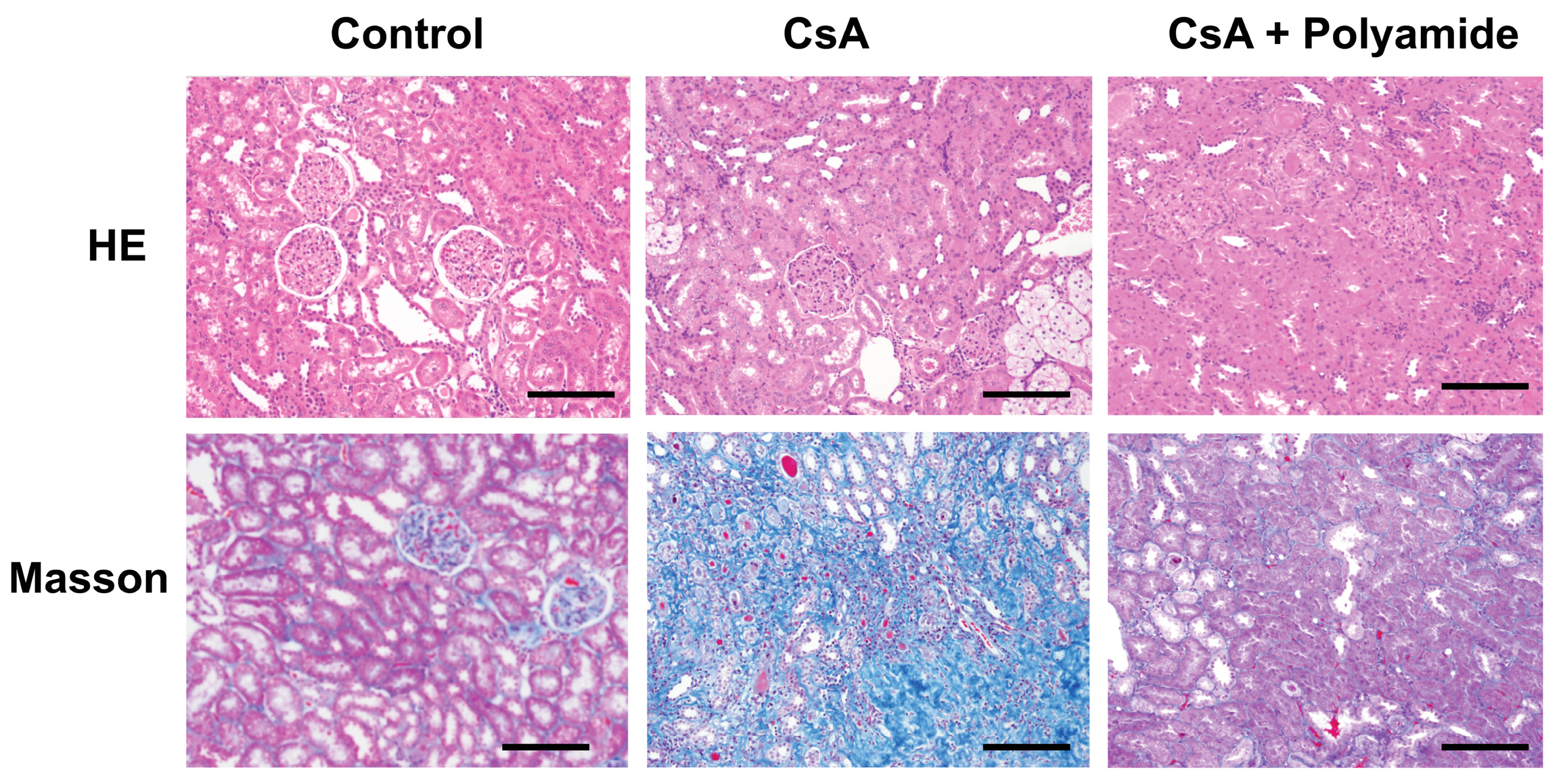
2.1. Effects of Polyamide on Fibrosis in Cyclosporine A (CsA)-Induced Nephropathy in Marmosets
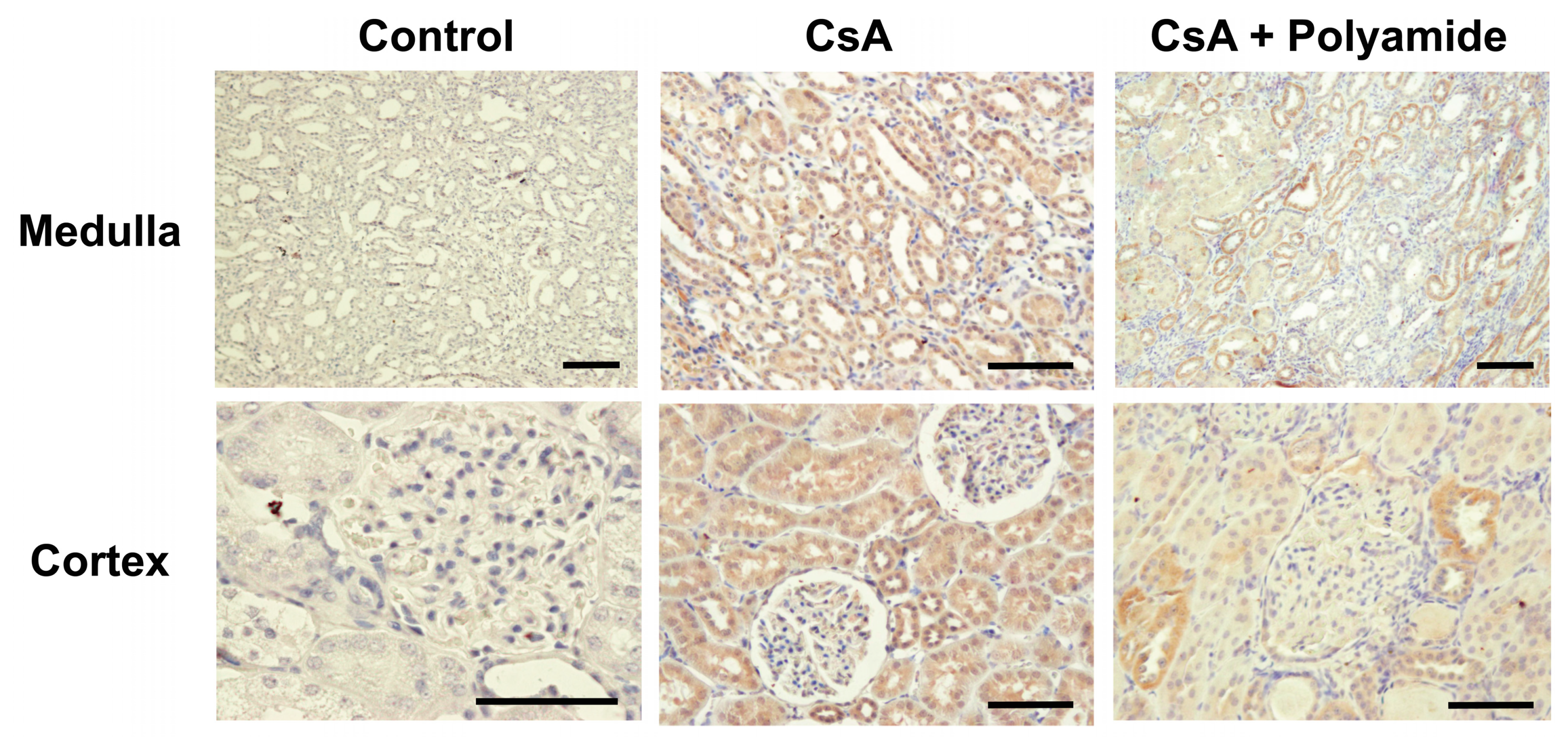
2.2. Effects of Polyamide on TGF-β1 Expression in CsA-Induced Nephropathy in Marmosets
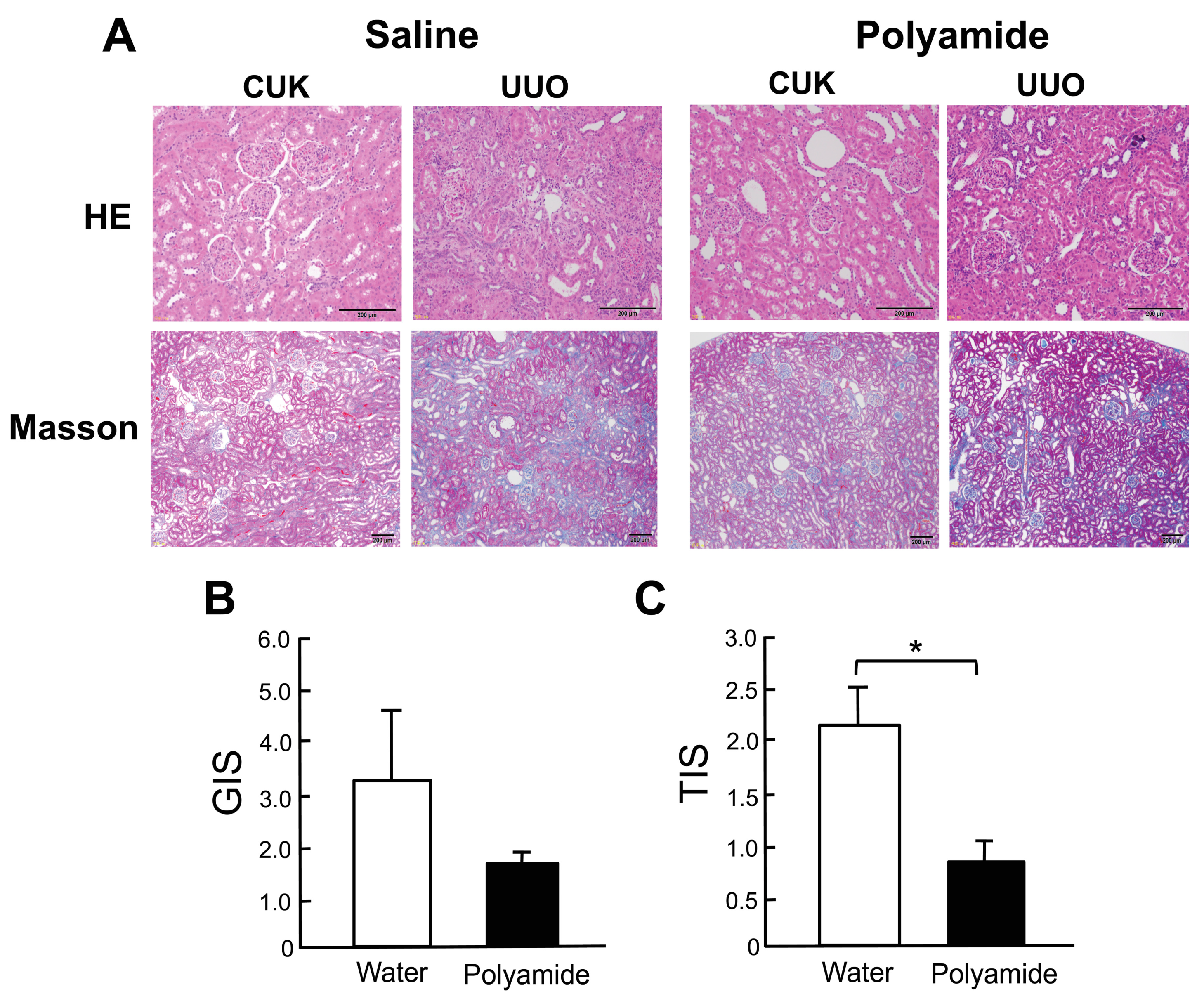
2.3. Effects of Polyamide on Renal Degeneration in Marmoset Kidneys with Unilateral Urethral Obstruction (UUO)
2.4. Effects of Polyamide on the Expression of TGF-β1, α-Smooth Muscle Actin (α-SMA), and E-cadherin in Marmoset Kidneys with UUO
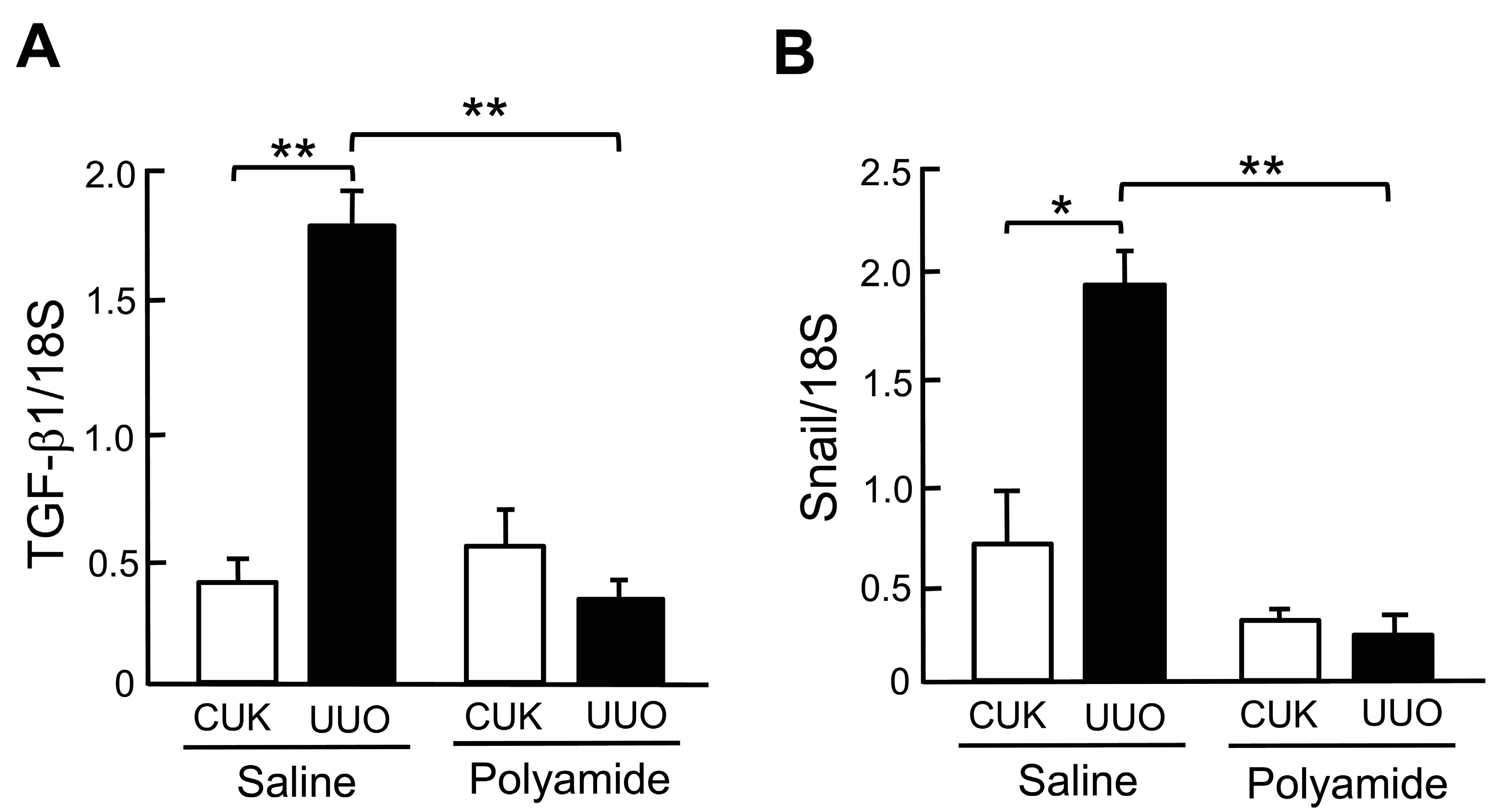
2.5. Effects of Polyamide on the Expression of TGF-β1 and Snail mRNAs in Marmoset Kidneys with UUO
3. Discussion
4. Materials and Methods
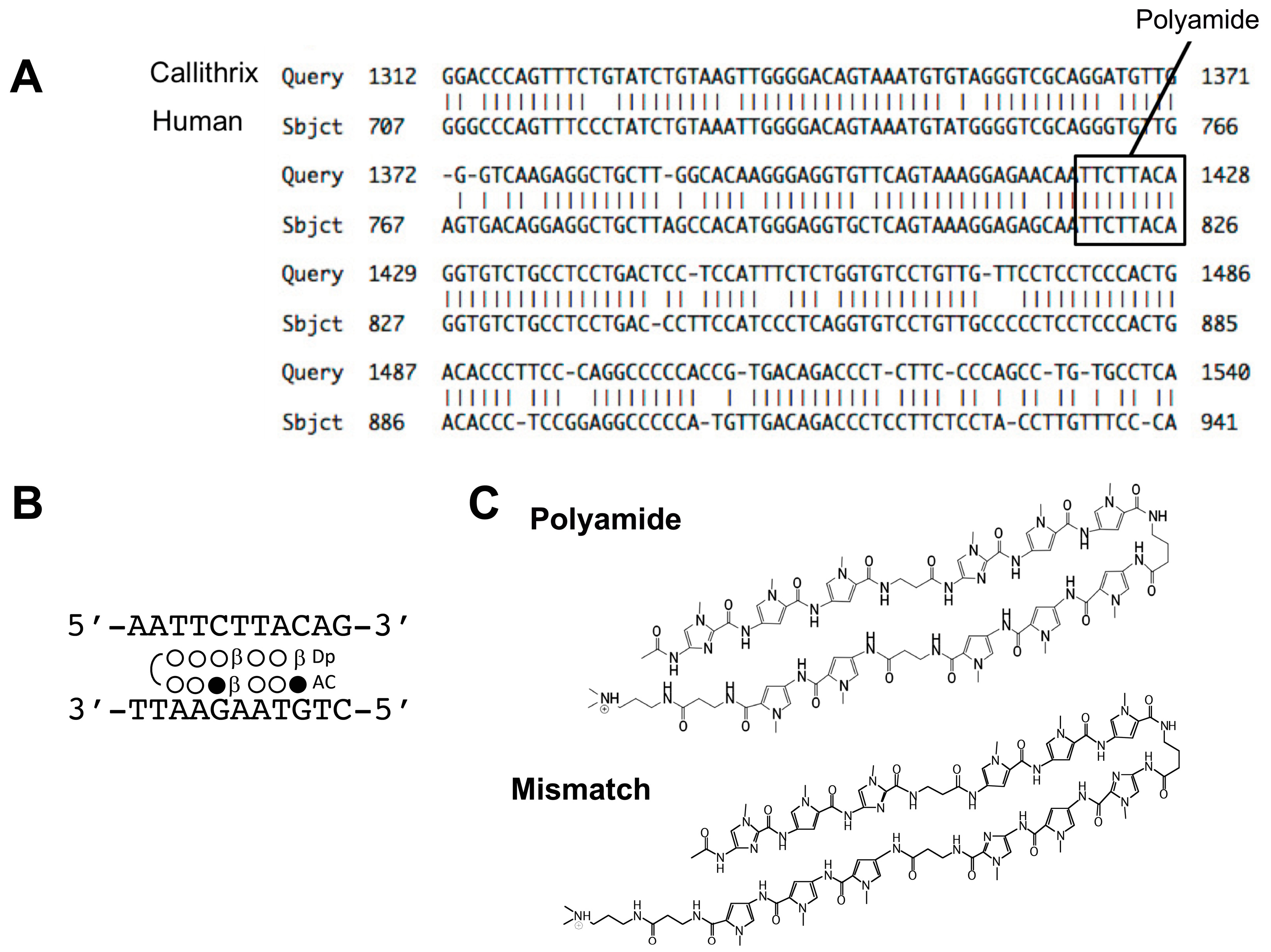
4.1. Design and Synthesis of PI Polyamides Targeting the hTGF-β1 Promoter
4.2. Cell Culture
4.3. RNA Extraction and Real-Time PCR
4.4. Ethics and Animals
4.5. Creation of Nephropathy in Marmosets and Treatment with PI Polyamides
4.6. Histopathological and Immunohistochemical Examinations
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trauger, J.W.; Baird, E.E.; Dervan, P.B. Recognition of DNA by designed ligands at subnanomolar concentrations. Nature 1996, 382, 559–561. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Baird, E.E.; Dervan, P.B. On the pairing rules for recognition in the minor groove of DNA by pyrrole-imidazole polyamides. Chem. Biol. 1997, 4, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Gottesfeld, J.M.; Neely, L.; Trauger, J.W.; Baird, E.E.; Dervan, P.B. Regulation of gene expression by small molecules. Nature 1997, 387, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.S.; Sugiyama, H. Biology of N-methylpyrrole-N-methylimidazole hairpin polyamide. Biol. Pharm. Bull. 2004, 27, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, A.; Fukuda, N.; Takahashi, T.; Watanabe, T.; Matsuda, H.; Nagase, H.; Bando, T.; Sugiyama, H.; Shimizu, K. RNA binding properties of novel gene silencing pyrrole-imidazole polyamides. Biol. Pharm. Bull. 2013, 36, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Iida, H.; Kawakami, M.; Bando, T.; Tao, Z.F.; Sugiyama, H. Sequence-specific protection of plasmid DNA from restriction endonuclease hydrolysis by pyrrole-imidazole-cyclopropapyrroloindole conjugates. Nucleic Acids Res. 2002, 30, 3748–3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sporn, M.B.; Roberts, A.B. Transforming growth factor-beta: Recent progress and new challenges. J. Cell. Biol. 1992, 119, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J.A.; Lyon, R.M.; Moses, H.L. The cell biology of transforming growth factor β. Biochem. Biophys. Acta 1990, 1032, 79–87. [Google Scholar] [CrossRef]
- Lai, Y.-M.; Fukuda, N.; Ueno, T.; Kishioka, H.; Matsuda, Y.; Matsuda, H.; Saito, S.; Matsumoto, K.; Ayame, H.; Bando, T.; et al. Synthetic pyrrole-imidazole polyamide inhibits expression of the human transforming growth factor-β1 gene. J. Pharmacol. Exp. Ther. 2005, 315, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Fukuda, N.; Ueno, T.; Tahira, Y.; Ayame, H.; Bando, T.; Sugiyama, H.; Saito, S.; Matsumoto, K.; Mugishima, H.; et al. Development of gene silencing pyrrole-imidazole polyamide targeted to the TGF-β promoter for treatment of progressive renal diseases. J. Am. Soc. Nephrol. 2006, 17, 422–432. [Google Scholar] [CrossRef]
- Matsuda, H.; Fukuda, N.; Ueno, T.; Katakawa, M.; Wang, X.; Watanabe, T.; Matsui, S.; Aoyama, T.; Saito, K.; Bando, T.; et al. Transcriptional inhibition of progressive renal disease by gene silencing pyrrole-imidazole polyamide targeting of the transforming growth factor-β1 promoter. Kidney Int. 2011, 79, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.-H.; Fukuda, N.; Ueno, T.; Matsuda, H.; Nagase, H.; Matsumoto, Y.; Sugiyama, H.; Matsumoto, K. A novel gene silencer pyrrole-imidazole polyamide targeting transforming growth factor-β1 inhibits restenosis and preserved endothelialization in the injured artery. Cardiovasc. Res. 2009, 81, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Matsuda, H.; Wang, L.; Watanabe, T.; Kimura, T.; Igarashi, J.; Wang, X.; Sakimoto, T.; Fukuda, N.; Sawa, M.; et al. Pretranscriptional regulation of Tgf-β1 by PI polyamide prevents scarring and accelerates wound healing of the cornea after exposure to alkali. Mol. Ther. 2010, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Washio, H.; Fukuda, N.; Matsuda, H.; Nagase, H.; Watanabe, T.; Matsumoto, Y.; Terui, T. Transcriptional inhibition of hypertrophic scars by a gene silencer, pyrrole-imidazole polyamide, targeting the TGF-β1 promoter. J. Investig. Dermatol. 2011, 31, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Inami, M.; Fukushima, A.; Ueno, T.; Yamada, T.; Tsunemi, A.; Matsumoto, Y.; Fukuda, N.; Soma, M.; Moriyama, M. Reduction of dimethylnitrosamine-induced liver fibrosis by the novel gene regulator PI polyamide targeting transforming growth factor β1 gene. Biol. Pharm. Bull. 2015, 38, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Serie, K.; Fukuda, N.; Nakai, S.; Matsuda, H.; Maruyama, T.; Murayama, Y.; Omata, S. Pyrrole-imidazole polyamide targeting transforming growth factor β1 ameliorates encapsulating peritoneal sclerosis. Perit. Dial. Int. 2012, 32, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Glicks, A.; Sporn, M.B.; Roberts, A. Characterization of the promoter region of the human transforming growth factor-β1 gene. J. Biol. Chem. 1989, 264, 402–408. [Google Scholar] [PubMed]
- Igarashi, J.; Fukuda, N.; Inoue, T.; Nakai, S.; Saito, K.; Fujiwara, K.; Matsuda, H.; Ueno, T.; Matsumoto, Y.; Watanabe, T.; et al. Preclinical study of novel gene silencer pyrrole-imidazole polyamide targeting human TGF-β1 promoter for hypertrophic scars in a common marmoset primate model. PLoS ONE 2015, 10, e0125295. [Google Scholar] [CrossRef]
- Benigni, A.; Morigi, M.; Perico, N.; Zoja, C.; Amuchastegui, C.S.; Piccinelli, A.; Donadelli, R.; Remuzzi, G. The acute effect of FK506 and cyclosporine on endothelial cell function and renal vascular resistance. Transplantation 1992, 54, 775–780. [Google Scholar] [CrossRef]
- Slattery, C.; Campbell, E.; McMorrow, T.; Ryan, M.P. Cyclosporine A-induced renal fibrosis: A role for epithelial-mesenchymal transition. Am. J. Pathol. 2005, 167, 395–407. [Google Scholar] [CrossRef]
- Ling, H.; Li, X.; Jha, S.; Wang, W.; Karetskaya, L.; Pratt, B.; Ledbetter, S. Therapeutic role of TGF-β-neutralizing antibody in mouse cyclosporin A nephropathy: Morphologic improvement associated with functional preservation. J. Am. Soc. Nephrol. 2003, 14, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Nakatani, T.; Yamanaka, S.; Tamada, S.; Kishimoto, T.; Tashiro, K.; Nakao, T.; Okamura, M.; Kim, S.; Iwao, H.; et al. Magnesium supplementation prevents experimental chronic cyclosporine a nephrotoxicity via renin-angiotensin system independent mechanism. Transplantation 2002, 74, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Osman, A.A.; Roggenbuck, D.; Mothes, T. IgA-gliadin antibodies, IgA-containing circulating immune complexes, and IgA glomerular deposits in wasting marmoset syndrome. Nephrol. Dial. Transplant. 1999, 14, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Bascands, J.L.; Schanstra, J.P. Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int. 2005, 68, 925–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneyama, T.; Kobayashi, S.; Aoyagi, D.; Ehara, T. Tranilast modulates fibrosis, epithelial-mesenchymal transition and peritubular capillary injury in unilateral ureteral obstruction rats. Pathology 2010, 42, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Aoyama, T.; Fukasawa, A.; Watabe, S.; Fukuda, N.; Ueno, T.; Sugiyama, H.; Nagase, H.; Matsumoto, Y. Determination of pyrrole-imidazole polyamide in rat plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2009, 877, 1070–1076. [Google Scholar] [CrossRef]
- Nagashima, T.; Aoyama, T.; Yokoe, T.; Fukasawa, A.; Fukuda, N.; Ueno, T.; Sugiyama, H.; Nagase, H.; Matsumoto, Y. Pharmacokinetic modeling and prediction of plasma pyrrole-imidazole polyamide concentration in rats using simultaneous urinary and biliary excretion data. Biol. Pharm. Bull. 2009, 32, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, M.; Bando, T.; Sasaki, S.; Shinohara, K.; Shimizu, T.; Fujimoto, J.; Sugiyama, H. DNA alkylation by pyrrole-imidazole seco-CBI conjugates with an indole linker: Sequence-specific DNA alkylation with 10-base-pair recognition through heterodimer formation. J. Am. Chem. Soc. 2007, 129, 5384–5390. [Google Scholar] [CrossRef]
- Taylor, R.D.; Asamitsu, S.; Takenaka, T.; Yamamoto, M.; Hashiya, K.; Kawamoto, Y.; Bando, T.; Nagase, H.; Sugiyama, H. Sequence-specific DNA alkylation targeting for Kras codon 13 mutation by pyrrole-imidazole polyamide seco-CBI conjugates. Chemistry 2014, 20, 1310–1317. [Google Scholar] [CrossRef]
- Hiraoka, K.; Inoue, T.; Taylor, R.D.; Watanabe, T.; Koshikawa, N.; Yoda, H.; Shinohara, K.; Takatori, A.; Sugimoto, H.; Maru, Y.; et al. Inhibition of KRAS codon 12 mutants using a novel DNA-alkylating pyrrole-imidazole polyamide conjugate. Nat. Commun. 2015, 6, 6706. [Google Scholar] [CrossRef]
- Shinohara, K.; Bando, T.; Sugiyama, H. Anticancer activities of alkylating pyrrole-imidazole polyamides with specific sequence recognition. Anticancer Drugs. 2010, 21, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Raij, L.; Azar, S.; Keane, W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984, 26, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuki, M.; Fukuda, N.; Inoue, T.; Mineshige, T.; Otsuki, T.; Horikoshi, S.; Endo, M.; Abe, M. Preclinical Study of DNA-Recognized Peptide Compound Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Progressive Renal Diseases in the Common Marmoset. Molecules 2019, 24, 3178. https://doi.org/10.3390/molecules24173178
Otsuki M, Fukuda N, Inoue T, Mineshige T, Otsuki T, Horikoshi S, Endo M, Abe M. Preclinical Study of DNA-Recognized Peptide Compound Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Progressive Renal Diseases in the Common Marmoset. Molecules. 2019; 24(17):3178. https://doi.org/10.3390/molecules24173178
Chicago/Turabian StyleOtsuki, Masari, Noboru Fukuda, Takashi Inoue, Takayuki Mineshige, Tomoyasu Otsuki, Shu Horikoshi, Morito Endo, and Masanori Abe. 2019. "Preclinical Study of DNA-Recognized Peptide Compound Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Progressive Renal Diseases in the Common Marmoset" Molecules 24, no. 17: 3178. https://doi.org/10.3390/molecules24173178





