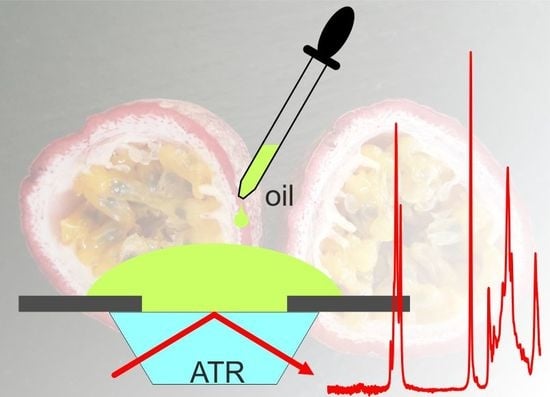
Identification of Passion Fruit Oil Adulteration by Chemometric Analysis of FTIR Spectra
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fatty Acid Profiles
2.2. The Spectrum of Passion Fruit Oil
2.3. Product Discrimination and Detection of Adulteration
2.4. Quantitative Analysis of Oil Mixtures
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Viganó, J.; Martínez, J. Trends for the application of passion fruit industrial by-products: A review on the chemical composition and extraction technique of phytochemicals. Food Public Health 2015, 5, 164–173. [Google Scholar] [CrossRef]
- Ferrari, R.A.; Colussi, F.; Ayub, R.A. Characterization of by-products of passion fruit industrialization utilization of seeds. Rev. Bras. Frutic. 2004, 26, 101–102. [Google Scholar] [CrossRef]
- Piombo, G.; Barouh, N.; Barea, B.; Boulanger, R.; Brat, P.; Pina, M.; Villeneuve, P. Characterization of the seed oils from kiwi (Actinidia chinensis), passion fruit (Passiflora eulis) and guava (Psidium guajava). Oléagineux Corps Gras Lipides 2006, 13, 195–199. [Google Scholar] [CrossRef]
- De Santana, F.C.; Shinagawa, F.B.; Araujo Eda, S.; Costa, A.M.; Mancini-Filho, J. Chemical Composition and Antioxidant Capacity of Brazilian Passiflora Seed Oils. Food Sci. 2015, 80, C2647–C2654. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, C.R.; Jorge, N. Yellow Passion Fruit Seed Oil (Passiflora edulis f. flavicarpa): Physical and Chemical Characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar] [CrossRef]
- Barrales, F.M.; Rezende, C.A.; Martínez, J. Supercritical CO2 extraction of passion fruit (Passiflora edulis sp.) seed oil assisted by ultrasound. J. Supercrit. Fluids 2015, 104, 183–192. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Sano, S.; Sugiyama, K.; Ito, T.; Katano, Y.; Ishihata, A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011, 59, 6209–6213. [Google Scholar] [CrossRef]
- Jorge, A.T.; Arroteia, K.F.; Santos, I.A.; Andres, E.; Medina, S.P.; Ferrari, C.R.; Lourenço, C.B.; Biaggio, R.M.; Moreira, P.L. Schinus terebinthifolius Raddi extract and linoleic acid from Passiflora edulis synergistically decrease melanin synthesis in B16 cells and reconstituted epidermis. Int. J. Cosmet. Sci. 2012, 34, 435–440. [Google Scholar] [CrossRef]
- Pereira, M.G.; Maciel, G.M.; Haminiuk, C.W.I.; Bach, F.; Hamerski, F.; de Paula Scheer, A.; Corazza, M.L. Effect of extraction process on composition, antioxidant and antibacterial activity of oil from yellow passion fruit (Passiflora edulis Var. Flavicarpa) seeds. Waste Biomass Valorization 2018, 10, 2611–2625. [Google Scholar] [CrossRef]
- Bezerra, C.V.; Rodrigues, A.M.D.C.; de Oliveira, P.D.; da Silva, D.A.; da Silva, L.H.M. Technological properties of amazonian oils and fats and their applications in the food industry. Food Chem. 2017, 221, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Banov, D.; Banov, F.; Bassani, A.S. Case Series: The Effectiveness of Fatty Acids from Pracaxi Oil in a Topical Silicone Base for Scar and Wound Therapy. Dermatol. Ther. 2014, 4, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.O.; Coppede, J.S.; Fernandes, V.C.; Sant’Ana, C.D.; Ticli, F.K.; Mazzi, M.V.; Giglio, J.R.; Pereira, P.S.; Soares, A.M.; Sampaio, S.V. Antihemorrhagic, antinucleolytic and other antiophidian properties of the aqueous extract from Pentaclethra macroloba. J. Ethnopharmacol. 2005, 100, 145–152. [Google Scholar] [CrossRef]
- Guclu, G.; Sevindik, O.; Kelebek, H.; Selli, S. Determination of Volatiles by Odor Activity Value and Phenolics of cv. Ayvalik Early-Harvest Olive Oil. Foods 2016, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for rapid authentication and detection of adulteration of food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caravaca, A.M.; Maggio, R.M.; Cerretani, L. Chemometric applications to assess quality and critical parameters of virgin and extra-virgin olive oil. A review. Anal. Chim. Acta 2016, 913, 1–21. [Google Scholar] [CrossRef]
- Rohman, A. The use of infrared spectroscopy in combination with chemometrics for quality control and authentication of edible fats and oils: A review. Appl. Spectr. Rev. 2017, 52, 589–604. [Google Scholar] [CrossRef]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Ferreira, B.S.; de Almeida, C.G.; Le Hyaric, M.; De Oliveira, V.E.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectroscopic investigation of carotenoids in oils from Amazonian products. Spectrosc. Lett. 2013, 46, 122–127. [Google Scholar] [CrossRef]
- Saraiva, S.A.; Cabral, E.C.; Eberlin, M.N.; Catharino, R.R. Amazonian vegetable oils and fats: Fast typification and quality control via triacyglycerol (TAG) profiles from dry matrix-assited laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry fingerprinting. J. Agric. Food Chem. 2009, 57, 4030–4034. [Google Scholar] [CrossRef]
- De Vasconcelos Viera Lopes, R.; Zamian, J.R.; Resck, I.S.; Araujo Sales, M.J.; dos Santos, M.L.; da Cunha, F.R. Physicochemical and rheological properties of passion fruit oil and its polyol. Eur. J. Lipid Sci. Technol. 2010, 112, 1253–1262. [Google Scholar] [CrossRef]
- Kiefer, J.; Bartels, J.; Kroll, S.; Rezwan, K. Vibrational spectroscopy as a promising toolbox for analyzing functionalized ceramic membranes. Appl. Spectrosc. 2018, 72, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Seidel, B.; Meyer, D. Optical Spectroscopy for the Analysis and Monitoring of Metalworking Fluids. Appl. Spectrosc. 2018, 72, 1790–1797. [Google Scholar] [CrossRef]
- Griffiths, P.R.; De Haseth, J.A. Fourier Transform Infrared Spectrometry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kiefer, J.; Frank, K.; Schuchmann, H.P. Attenuated total reflection infrared (ATR-IR) spectroscopy of a water-in-oil emulsion. Appl. Spectrosc. 2011, 65, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Pearson, K. Principal components analysis. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 6, 559. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal Component Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Kiefer, J.; Cromwell, A.L. Analysis of Scotch single malt whiskies using Raman spectroscopy. Anal. Methods 2017, 9, 511–518. [Google Scholar] [CrossRef]
- Durazzo, A.; Kiefer, J.; Lucarini, M.; Marconi, S.; Lisciani, S.; Camilli, E.; Gambelli, L.; Gabrielli, P.; Aguzzi, A.; Finotti, E.; et al. An Innovative and Integrated Food Research Approach: Spectroscopy applications to milk and a case study of a milk-based dish. Braz. J. Anal. Chem. 2018, 5, 12–27. [Google Scholar] [CrossRef]
- Durazzo, A.; Kiefer, J.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Aguzzi, A.; Gambelli, L.; Lisciani, S.; Marletta, L. Qualitative Analysis of Traditional Italian Dishes: FTIR Approach. Sustainability 2018, 10, 4112. [Google Scholar] [CrossRef]
- Bakeev, K.A. Process Analytical Technology, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Hair, J.F., Jr.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Pearson Educational Limited: Essex, UK, 2014. [Google Scholar]
- Noack, K.; Eskofier, B.; Kiefer, J.; Dilk, C.; Bilow, G.; Schirmer, M.; Buchholz, R.; Leipertz, A. Combined shifted-excitation Raman difference spectroscopy and support vector regression for monitoring the algal production of complex polysaccharides. Analyst 2013, 138, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.W. Prozessanalytik, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Nyanzi, S.A.; Carstensen, B.; Schwack, W. A comparative study of acid profiles of Passiflora seed oils from Uganda. J. Am. Oil Chem. Soc. 2005, 82, 41–44. [Google Scholar] [CrossRef]
- Rana, V.S.; Blazquez, A.M. Fatty Acid Composition of Passiflora edulis Sims. Seed Oil. J. Lipid Sci. Technol. 2008, 40, 65–66. [Google Scholar]
- Codex Alimentarius: Fats, Oils and Related Products, 2nd ed.; Food and Agricultural Organization of the United Nations World Health Organization: Rome, Italy, 2001; Volume 8.
- Lucarini, M.; Durazzo, A.; Raffo, A.; Giovannini, A.; Kiefer, J. Passion Fruit (Passiflora spp.) Seed Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M., Ed.; Springer: Heidelberg/Berlin, Germany, 2019; pp. 577–603. [Google Scholar]
- Lampe, A.I.; Dittmar, A.K.; Heyen, C.; Kiefer, J. Butanol as a potential biofuel: A spectroscopic study of its blends with n-decane and diesel. Fuel 2018, 222, 312–318. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E.D. Red-, blue-, or no-shift in hydrogen bonds: A unified explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Noack, K.; Bartelmess, J.; Walter, C.; Dörnenburg, H.; Leipertz, A. Vibrational structure of the polyunsaturated fatty acids eicosapentaenoic acid and arachidonic acid studied by infrared spectroscopy. J. Mol. Struct. 2010, 965, 121–124. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are generally available from the authors, but we note that the “best before” dates of some of them have already expired at the time the article has been published. |








| Fatty Acid | PF1 | PF2 | PF3 | PF4 | PF5 | Typical Range |
|---|---|---|---|---|---|---|
| C14:0 | 0.08 (0.07) | 0.05 (0.05) | 0.07 (0.07) | 0.00 (0.00) | 0.07 (0.06) | <0.2% |
| C16:0 | 12.90 (0.50) | 8.39 (0.55) | 12.36 (0.88) | 8.06 (0.49) | 12.50 (0.21) | 8–11% |
| C18:0 | 3.50 (0.06) | 2.74 (0.48) | 2.49 (0.28) | 2.55 (0.57) | 2.62 (0.05) | 1–4% |
| C18:1 | 24.79 (0.56) | 24.44 (1.54) | 16.57 (0.87) | 25.46 (1.36) | 16.09 (0.41) | 13–17% |
| C18:2 | 52.17 (0.54) | 63.26 (2.25) | 68.30 (0.57) | 63.71 (2.38) | 68.30 (0.54) | 67–74% |
| C18:3 | 6.56 (0.88) | 1.12 (0.24) | 0.20 (0.19) | 0.22 (0.03) | 0.41 (0.05) | <0.5% |
| ΣUFA 1 | 83.52 (1.98) | 88.82 (4.03) | 85.07 (1.36) | 89.39 (3.77) | 84.88 (1.00) | |
| ΣMUFA 2 | 24.79 (0.56) | 24.44 (1.54) | 16.57 (0.87) | 25.46 (1.36) | 16.09 (0.41) | |
| ΣPUFA 3 | 58.73 (1.42) | 64.38 (2.49) | 68.50 (0.76) | 63.93 (2.41) | 68.71 (0.59) |
| Fatty Acid | Sunflower | Rapeseed | Olive |
|---|---|---|---|
| C14:0 | <0.2% | <0.2% | <0.1% |
| C16:0 | 5.0–7.6% | 1.5–6.0% | 7.5–20.0% |
| C18:0 | 2.7–6.5% | 0.5–3.1% | 0.5–5.0% |
| C18:1 | 14.0–39.4% | 8–60% | 55–83% |
| C18:2 | 48.8–74.0% | 11–23% | 3.5–21.0% |
| C18:3 | <0.3% | 5–13% | <1.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, J.; Lampe, A.I.; Nicoli, S.F.; Lucarini, M.; Durazzo, A. Identification of Passion Fruit Oil Adulteration by Chemometric Analysis of FTIR Spectra. Molecules 2019, 24, 3219. https://doi.org/10.3390/molecules24183219
Kiefer J, Lampe AI, Nicoli SF, Lucarini M, Durazzo A. Identification of Passion Fruit Oil Adulteration by Chemometric Analysis of FTIR Spectra. Molecules. 2019; 24(18):3219. https://doi.org/10.3390/molecules24183219
Chicago/Turabian StyleKiefer, Johannes, Anja I. Lampe, Stefano F. Nicoli, Massimo Lucarini, and Alessandra Durazzo. 2019. "Identification of Passion Fruit Oil Adulteration by Chemometric Analysis of FTIR Spectra" Molecules 24, no. 18: 3219. https://doi.org/10.3390/molecules24183219