Fluorine-Substituted Arylphosphine for an NHC-Ni(I) System, Air-Stable in a Solid State but Catalytically Active in Solution
Abstract
:1. Introduction
2. Results
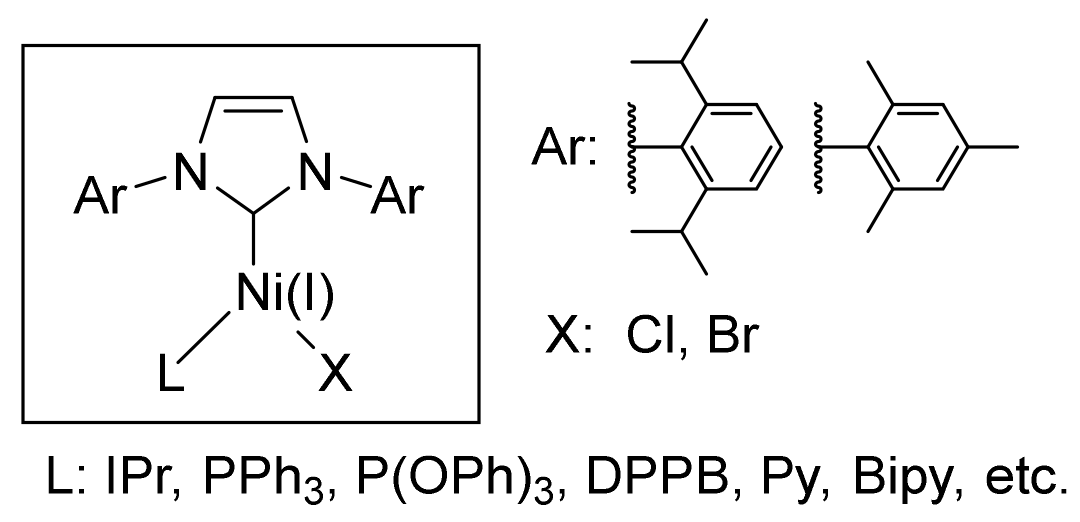
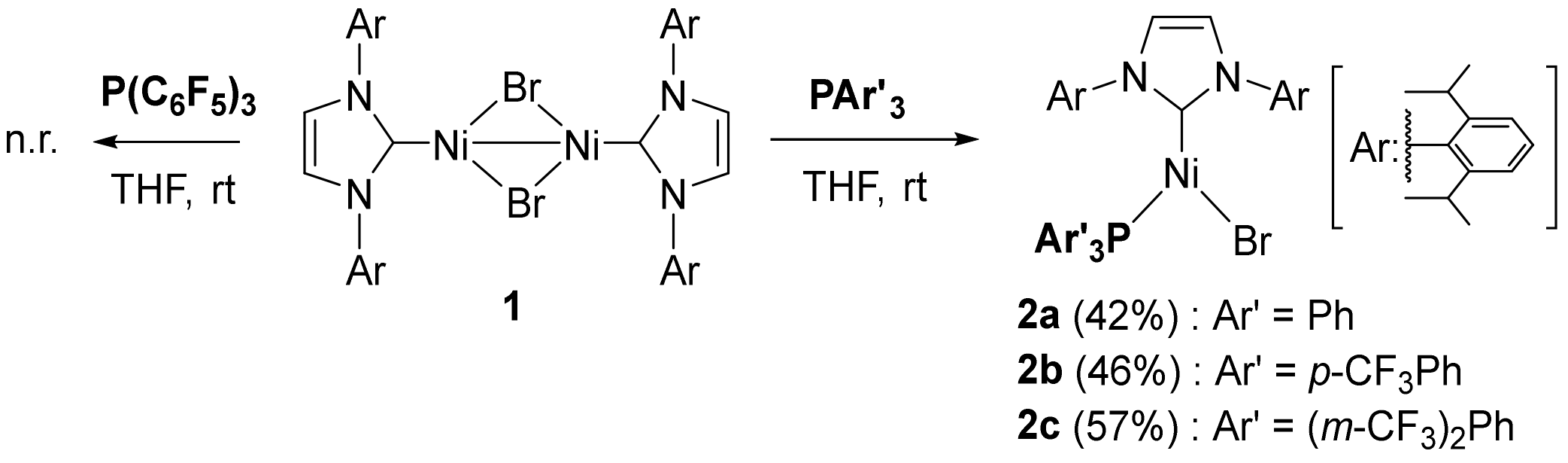
2.1. Preparation and Characterization of Ni(I) Complexes
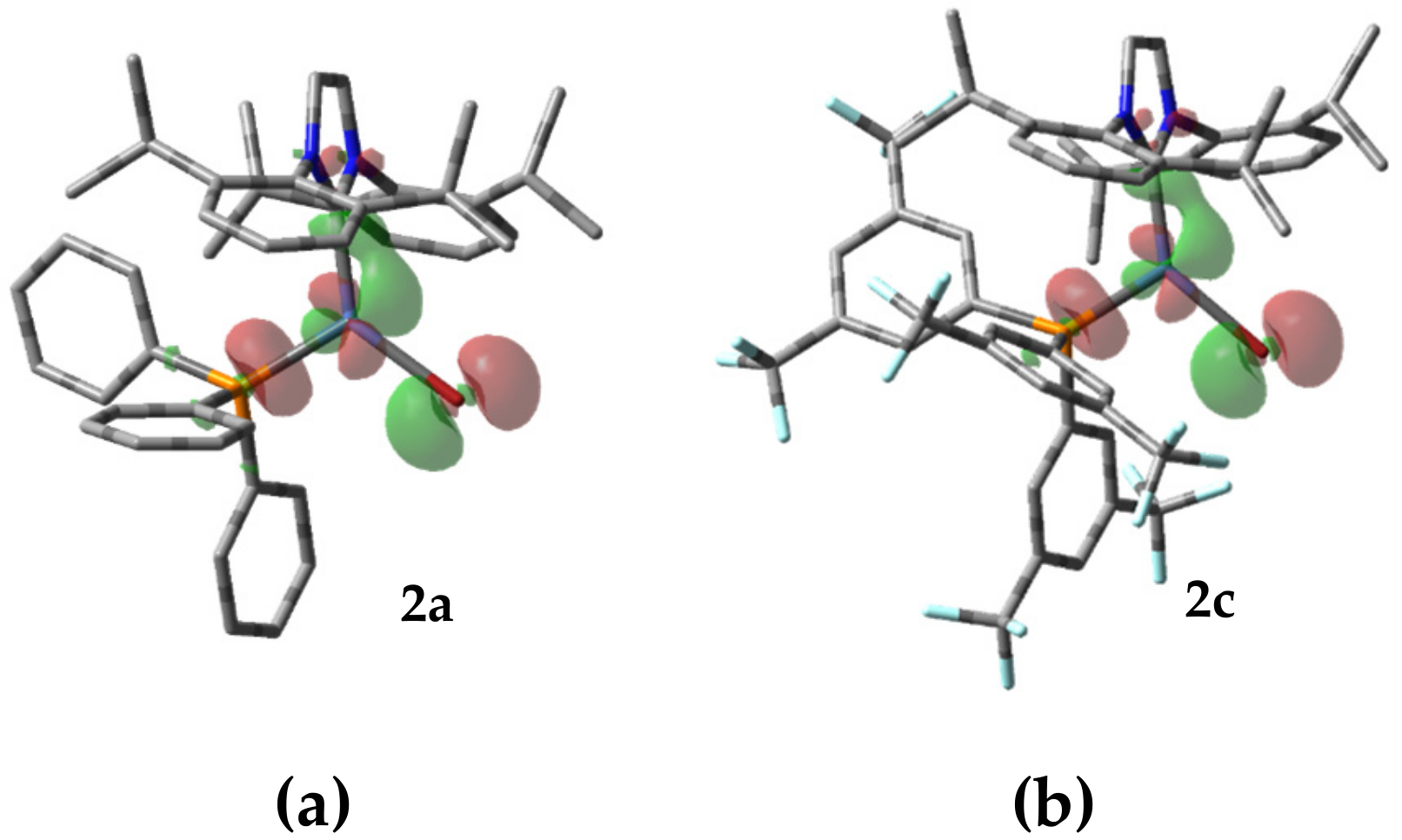
2.2. X-ray Crystallography and Theoretical Studies
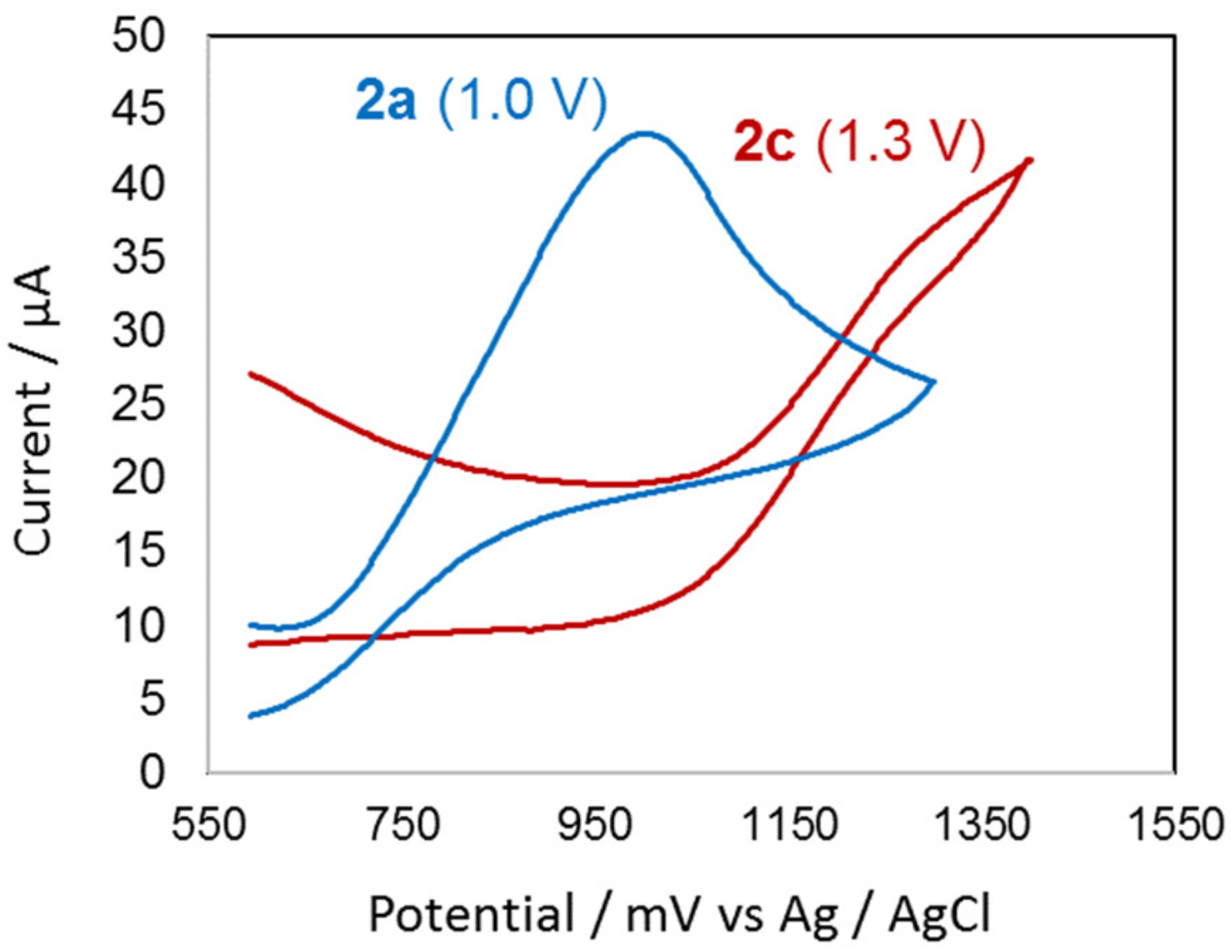
2.3. Oxidation Potentials
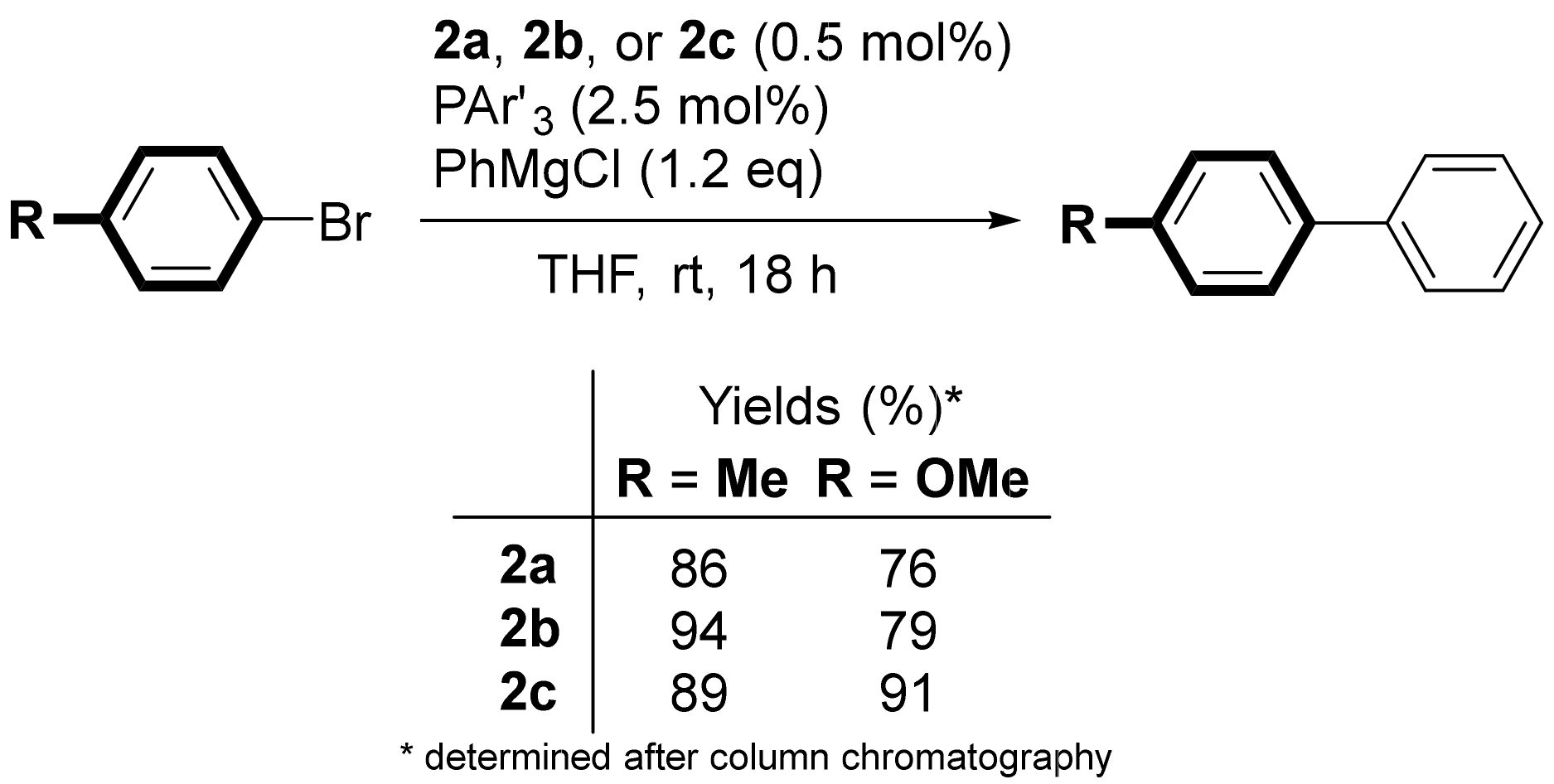
2.4. Catalytic Performance for Cross-Coupling Reaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Ni(IPr)(PPh3)Br (2a)
4.1.2. Ni(IPr)(PAr(p-CF3)3)Br (2b)
4.1.3. Ni(IPr)(PAr(m-CF3)3)Br (2c)
4.2. Methods
4.2.1. Kumada–Tamao–Corriu Coupling of Aryl Bromides
4.2.2. X-ray Crystallography
4.2.3. Theoretical Details
4.2.4. Electrochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tasker, S.Z.; Stanley, E.A.; Jamison, T.F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Wang, X.; Xue, W.; Gong, H. Nickel-catalyzed reductive coupling of alkyl halides with other electrophiles: Concept and mechanistic considerations. Org. Chem. Front. 2015, 2, 1411–1421. [Google Scholar] [CrossRef]
- Tobisu, M.; Chatani, N. Cross-Couplings Using Aryl Ethers via C–O Bond Activation Enabled by Nickel Catalysts. Acc. Chem. Res. 2015, 48, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Ritleng, V.; Henrion, M.; Chetcuti, M.J. Nickel N-Heterocyclic Carbene-Catalyzed C–Heteroatom Bond Formation, Reduction, and Oxidation: Reactions and Mechanistic Aspects. ACS Catal. 2016, 6, 890–906. [Google Scholar] [CrossRef]
- Zimmermann, P.; Limberg, C. Activation of Small Molecules at Nickel(I) Moieties. J. Am. Chem. Soc. 2017, 139, 4233–4242. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, T.; Koga, Y.; Matsubara, K. Dinuclear Nickel(I) and Palladium(I) Complexes for Highly Active Transformations of Organic Compounds. Molecules 2018, 23, 140. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Power, P.P. Complexes of Ni(I): A “rare” oxidation state of growing importance. Chem. Soc. Rev. 2017, 46, 5347–5399. [Google Scholar] [CrossRef] [PubMed]
- Schley, N.D.; Fu, G.C. Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J. Am. Chem. Soc. 2014, 136, 16588–16593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, S.; Koga, Y.; Matsumoto, T.; Matsubara, K. A new aspect of nickel-catalyzed Grignard cross-coupling reactions: Selective synthesis, structure, and catalytic behavior of a T-shape three-coordinate nickel(I) chloride bearing a bulky NHC ligand. Chem. Commun. 2010, 46, 1932–1934. [Google Scholar] [CrossRef]
- Davies, C.J.E.; Page, M.J.C.; Ellul, E.; Mahon, M.F.; Whittlesey, M.K. Ni(I) and Ni(II) ring-expanded N-heterocyclic carbene complexes: C–H activation, indole elimination and catalytic hydrodehalogenation. Chem. Commun. 2010, 46, 5151–5153. [Google Scholar] [CrossRef]
- Zhang, K.; Conda-Sheridan, M.; Cooke, S.R.; Louie, J. N-Heterocyclic Carbene Bound Nickel(I) Complexes and Their Roles in Catalysis. Organometallics 2011, 30, 2546–2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.; Kaiser, A.; Sarkar, B.; Wanner, M.; Fiedler, J. The Electrochemical Behaviour of Organonickel Complexes: Mono-, Di- and Trivalent Nickel. Eur. J. Inorg. Chem. 2007, 965–976. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Liu, Y. Nickel-catalyzed cyclization of alkyne-nitriles with organoboronic acids involving anti-carbometalation of alkynes. Chem. Sci. 2016, 7, 5815–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.-C.; Xie, S.-J.; Fang, H.; Shi, Z.-J. Ni-Catalyzed Cross-Coupling of Dimethyl Aryl Amines with Arylboronic Esters under Reductive Conditions. J. Am. Chem. Soc. 2018, 140, 13575–13579. [Google Scholar] [CrossRef]
- Szatkowski, L.; Hall, M.B. Dehalogenation of chloroalkanes by nickel(I) porphyrin derivatives, a computational study. Dalton Trans. 2016, 45, 16869–16877. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-H.; Kudisch, M.; Liu, B.; Miyake, G.M. C–N Cross-Coupling via Photoexcitation of Nickel–Amine Complexes. J. Am. Chem. Soc. 2018, 140, 7667–7673. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Phillips, D.L. Density Functional Theory Studies of Negishi Alkyl–Alkyl Cross-Coupling Reactions Catalyzed by a Methylterpyridyl-Ni(I) Complex. J. Org. Chem. 2008, 73, 3680–3688. [Google Scholar] [CrossRef] [PubMed]
- Phapale, V.B.; Guisán-Ceinos, M.; Buñuel, E.; Cárdenas, D.J. Ni-Catalyzed Cascade Cyclization–Kumada Alkyl–Alkyl Cross-Coupling. Chem. Eur. J. 2009, 15, 12681–12688. [Google Scholar] [CrossRef]
- Jones, G.D.; Martin, J.L.; McFarland, C.; Allen, O.R.; Hall, R.E.; Haley, A.D.; Brandon, R.J.; Konovalova, T.; Desrochers, P.J.; Pulay, P.; et al. Ligand redox effects in the synthesis, electronic structure, and reactivity of an alkyl-alkyl cross-coupling catalyst. J. Am. Chem. Soc. 2006, 128, 13175–13183. [Google Scholar] [CrossRef]
- Ren, H.; Li, G.-F.; Zhu, B.; Lv, X.-D.; Yao, L.-S.; Wang, X.-L.; Su, Z.-M.; Guan, W. How Does Iridium(III) Photocatalyst Regulate Nickel(II) Catalyst in Metallaphotoredox-Catalyzed C–S Cross-Coupling? Theoretical and Experimental Insights. ACS Catal. 2019, 9, 3858–3865. [Google Scholar] [CrossRef]
- Inatomi, T.; Fukahori, Y.; Yamada, Y.; Ishikawa, R.; Kanegawa, S.; Koga, Y.; Matsubara, K. Ni(I)–Ni(III) cycle in Buchwald–Hartwig amination of aryl bromide mediated by NHC-ligated Ni(I) complexes. Cat. Sci. Technol. 2019, 9, 1784–1793. [Google Scholar] [CrossRef]
- Zarate, C.; Yang, H.; Bezdek, M.J.; Hesk, D.; Chirik, P.J. Ni(I)-X Complexes Bearing a Bulky α-Diimine Ligand: Synthesis, Structure, and Superior Catalytic Performance in the Hydrogen Isotope Exchange in Pharmaceuticals. J. Am. Chem. Soc. 2019, 141, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Charboneau, D.J.; Brudwig, G.W.; Hazari, N.; Lant, H.M.C.; Saydjari, A.K. Development of an Improved System for the Carboxylation of Aryl Halides through Mechanistic Studies. ACS Catal. 2019, 9, 3228–3241. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.A.S.; Lovatt, J.D.; McArdle, P.; Cunningham, D.; Mainome, E.; Gottlieb, H.E.; Goldschmidt, Z. The effect of fluorine, trifluoromethyl and related substitution on the donor properties of triarylphosphines towards [Fe(CO)4]. Inorg. Chem. Commun. 1998, 1, 118–120. [Google Scholar] [CrossRef]
- Howell, J.A.S.; Fey, N.; Lovatt, J.D.; Yates, P.C.; McArdle, P.; Hursthouse, M.B.; Light, M.E. Effect of fluorine and trifluoromethyl substitution on the donor properties and stereodynamical behaviour of triarylphosphines. J. Chem. Soc. Dalton Trans. 1999, 17, 3015–3028. [Google Scholar] [CrossRef]
- Zuccaccia, D.; Belpassi, L.; Rocchigiani, L.; Tarantelli, F.; Macchioni, A. A Phosphine Gold(I) π-Alkyne Complex: Tuning the Metal−Alkyne Bond Character and Counterion Position by the Choice of the Ancillary Ligand. Inorg. Chem. 2010, 49, 3080–3082. [Google Scholar] [CrossRef]
- Kolter, M.; Böck, K.; Karaghiosoff, K.; Koszinowski, K. Anionic Palladium(0) and Palladium(II) Ate Complexes. Angew. Chem. Int. Ed. 2017, 56, 13244–13248. [Google Scholar] [CrossRef]
- Cutillas, N.; Martínez, A.; Yellol, G.S.; Rodríguez, V.; Zamora, A.; Pedreño, M.; Donaire, A.; Janiak, C.; Ruiz, J. Anticancer C,N-Cycloplatinated(II) Complexes Containing Fluorinated Phosphine Ligands: Synthesis, Structural Characterization, and Biological Activity. Inorg. Chem. 2013, 52, 13529–13535. [Google Scholar] [CrossRef]
- Jakab, A.; Dalicsek, Z.; Holczbauer, T.; Hamza, A.; Pápai, I.; Finta, Z.; Timári, G.; Soós, T. Superstable Palladium(0) Complex as an Air- and Thermostable Catalyst for Suzuki Coupling Reactions. Eur. J. Org. Chem. 2015, 1, 60–66. [Google Scholar] [CrossRef]
- Korenaga, T.; Ko, A.; Uotani, K.; Tanaka, Y.; Sakai, T. Synthesis and Application of 2,6-Bis(trifluoromethyl)-4-pyridyl Phosphanes: The Most Electron-Poor Aryl Phosphanes with Moderate Bulkiness. Angew. Chem. Int. Ed. 2011, 50, 10703–10707. [Google Scholar] [CrossRef]
- Stark, M.J.; Shaw, M.J.; Fadamin, A.; Rath, N.P. Synthesis, structural characterization and catalytic activity of indenyl complexes of ruthenium bearing fluorinated phosphine ligands. J. Organomet. Chem. 2017, 847, 41–53. [Google Scholar] [CrossRef]
- Dible, B.R.; Sigman, M.S.; Arif, A.M. Oxygen-Induced Ligand Dehydrogenation of a Planar Bis-μ-Chloronickel(I) Dimer Featuring an NHC Ligand. Inorg. Chem. 2005, 44, 3774–3776. [Google Scholar] [CrossRef] [PubMed]
- Nagao, S.; Matsumoto, T.; Koga, Y.; Matsubara, K. Monovalent Nickel Complex Bearing a Bulky N-Heterocyclic Carbene Catalyzes Buchwald-Hartwig Amination of Aryl Halides under Mild Conditions. Chem. Lett. 2011, 40, 1036–1038. [Google Scholar] [CrossRef]
- Matsubara, K.; Fukahori, Y.; Inatomi, T.; Tazaki, S.; Yamada, Y.; Koga, Y.; Kanegawa, S.; Nakamura, T. Monomeric Three-Coordinate N-Heterocyclic Carbene Nickel(I) Complexes: Synthesis, Structures, and Catalytic Applications in Cross-Coupling Reactions. Organometallics 2016, 35, 3281–3287. [Google Scholar] [CrossRef]
- Laskowski, C.A.; Bungum, D.J.; Baldwin, S.M.; Del Ciello, S.A.; Iluc, V.M.; Hillhouse, G.L. Synthesis and Reactivity of Two-Coordinate Ni(I) Alkyl and Aryl Complexes. J. Am. Chem. Soc. 2013, 135, 18272–18275. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.L.; Ellis, D.; Mason, K.L.; Orpen, A.G.; Pringle, P.G.; Wingad, R.L.; Zaher, D.A.; Baker, R.T. The electron-poor phosphines P{C6H3(CF3)2-3,5}3 and P(C6F5)3 do not mimic phosphites as ligands for hydroformylation. A comparison of the coordination chemistry of P{C6H3(CF3)2-3,5}3 and P(C6F5)3 and the unexpectedly low hydroformylation activity of their rhodium complexes. Dalton Trans. 2005, 7, 1294–1300. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 (Revision A.03); Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hasegawa, T. Understanding of the intrinsic difference between normal- and perfluoro-alkyl compounds toward total understanding of material properties. Chem. Phys. Lett. 2015, 627, 64–66. [Google Scholar] [CrossRef] [Green Version]
- Pollice, R.; Chen, P. Origin of the immiscibility of alkanes and perfluoroalkanes. J. Am. Chem. Soc. 2019, 141, 3489–3506. [Google Scholar] [CrossRef]
- Jafarpour, L.; Stevens, E.D.; Nolan, S.P. A sterically demanding nucleophilic carbene: 1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene). Thermochemistry and catalytic application in olefin metathesis. J. Organomet. Chem. 2000, 606, 49–54. [Google Scholar] [CrossRef]
- Crystal Structure; Version. 4.2: Crystal Structure Analysis Package; Rigaku Co.: Tokyo, Japan, 2015; pp. 196–8666.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| 2a | 2c | ||
|---|---|---|---|
| Lengths (Å) | Ni(1)–Br(1) | 2.301(1) | 2.3016(8) |
| Ni(1)–P(1) | 2.201(1) | 2.182(1) | |
| Ni(1)–C(1) | 1.918(5) | 1.936(3) | |
| Angles (°) | C(1)–Ni(1)–Br(1) | 138.4(1) | 137.1(1) |
| C(1)–Ni(1)–P(1) | 114.9(1) | 113.5(1) | |
| Br(1)–Ni(1)–P(1) | 106.44(4) | 109.29(4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, K.; Fujii, T.; Hosokawa, R.; Inatomi, T.; Yamada, Y.; Koga, Y. Fluorine-Substituted Arylphosphine for an NHC-Ni(I) System, Air-Stable in a Solid State but Catalytically Active in Solution. Molecules 2019, 24, 3222. https://doi.org/10.3390/molecules24183222
Matsubara K, Fujii T, Hosokawa R, Inatomi T, Yamada Y, Koga Y. Fluorine-Substituted Arylphosphine for an NHC-Ni(I) System, Air-Stable in a Solid State but Catalytically Active in Solution. Molecules. 2019; 24(18):3222. https://doi.org/10.3390/molecules24183222
Chicago/Turabian StyleMatsubara, Kouki, Takahiro Fujii, Rion Hosokawa, Takahiro Inatomi, Yuji Yamada, and Yuji Koga. 2019. "Fluorine-Substituted Arylphosphine for an NHC-Ni(I) System, Air-Stable in a Solid State but Catalytically Active in Solution" Molecules 24, no. 18: 3222. https://doi.org/10.3390/molecules24183222







