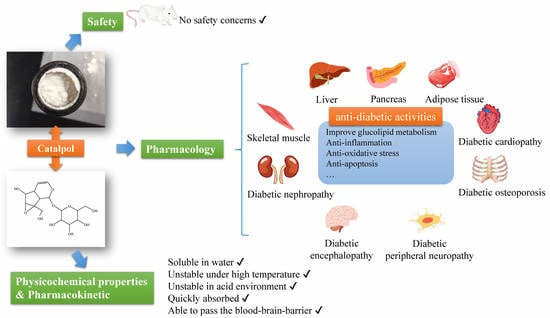
Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety
Abstract
:1. Introduction
2. Physicochemical Properties of Catalpol
3. Pharmacological Actions of Catalpol in the Management of Diabetes and Diabetic Complications
3.1. The Hypoglycemia Effect of Catalpol in Diabetic Animal Models
3.2. Catalpol and Liver
3.3. Catalpol and Pancreatic Tissue
3.4. Catalpol in Adipose Tissue
3.5. Catalpol in Skeletal Muscle Tissue
3.6. Catalpol and Diabetic Nephrology (DN)
3.7. Catalpol and Diabetic Cardiopathy
3.8. Catalpol and Diabetic Encephalopathy (DE)
3.9. Catalpol and Diabetic Peripheral Neuropathy (DPN)
3.10. Catalpol and Diabetic Osteoporosis
4. Pharmacokinetics of Catalpol
5. Safety Concern of Catalpol
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Ronsted, N.; Gobel, E.; Franzyk, H.; Jensen, S.R.; Olsen, C.E. Chemotaxonomy of Plantago. Iridoid glucosides and caffeoyl phenylethanoid glycosides. Phytochemistry 2000, 55, 337–348. [Google Scholar] [CrossRef]
- Søren, D. Biosynthesis of catalpol. Phytochemistry 1994, 35, 1187–1189. [Google Scholar]
- Tang, W.C.; Eisenbrand, G. Chinese Drugs of Plant Origin; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Kitagawa, I.; Nishimura, T.; Furubayashi, A.; Yosioka, I. On the constituents of rhizome of Rehmannia glutinosa Libosch. forma hueichingensis Hsiao. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1971, 91, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Meng, X.L. Research progress on chemical constituents and pharmacological activities of Rehmannia glutinosa. Drug Eval. Res. 2015, 38, 218–228. [Google Scholar]
- Wang, F.Q.; Xie, C.X.; Sun, R.B.; Zhang, Z.Y. Progress on germplasm enhancement and breeding of Rehmannia glutinosa. China J. Chin. Mater. Med. 2018, 43, 4203–4209. [Google Scholar]
- Zhang, H.M.; Liang, F.X.; Chen, R. Ancient records and modern research on the mechanisms of chinese herbal medicines in the treatment of diabetes mellitus. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Ni, Q. Traditional Chinese Medicine for Prevention and Treatment of Diabetes Mellitus under the Background of Precision Medicine. J. Med. Res. 2018, 47, 1–5. [Google Scholar]
- Wildman, R.P.; Gu, D.; Muntner, P.; Wu, X.; Reynolds, K.; Duan, X.; Chen, C.S.; Huang, G.; Bazzano, L.A.; He, J. Trends in overweight and obesity in Chinese adults: Between 1991 and 1999–2000. Obesity 2008, 16, 1448–1453. [Google Scholar] [CrossRef]
- American Diabetes Association (ADA). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 2018, 271–281. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, J.; Chan, P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.T.; Jia, W.Q.; Qiao, Y.Y.; Wei, Y. Determination of Catalpol in Tibetan Herb-medicine Lancea tibetica by RP-HPLC. Chin. Arch. Tradit. Chin. Med. 2017, 35, 2309–2311. [Google Scholar]
- Yu, H.H.; Zhong, M.; Ding, R.; Song, T.; Michiyo, Y.; Xu, X.Y.; Zheng, Y.M. A quantitative method for simultaneous assay of seven active ingredients with one marker in Scrophularia ningpoensis root. China J. Chin. Mater. Med. 2017, 42, 2719–2724. [Google Scholar]
- Dongyan, G.; Yanqiong, S.; Xing, D.; Zhao, L.; Xiaoli, L. Purification process of iridoid glycosides from the water-extraction of radix rehmanniae. J. Chang. Univ. Tradit. Chin. Med. 2014, 30, 1019–1021. [Google Scholar]
- Dang, J.L.; Guo, D.Y.; Shi, Y.J.; Wang, L. Opitimization of extraction technology for iridoid glycosides from Radix Rehmanniae by response surface methodology and its thermal stability. Cent. South. Pharm. 2017, 15, 745–749. [Google Scholar]
- Liu, Z.Q.; Yang, X.; Wang, T.; Zhu, H.F.; Dong, W. Pharmacokinetics of Different Administration Routes of Catalpol. J. Southwest Univ. 2014, 36, 222–226. [Google Scholar]
- Li, X.E.; Lin, Y.S.; Shan, Y.J. Study on content of catalpol in varieties and earthnuts of Rehmannia glutinosa. Chin. Pharm. J. 2002, 37, 20–23. [Google Scholar]
- Wang, Y.; Liao, D.; Qin, M.; Li, X.E. Simultaneous Determination of Catalpol, Aucubin, and Geniposidic Acid in Different Developmental Stages ofRehmannia glutinosaLeaves by High Performance Liquid Chromatography. J. Anal. Methods Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Dai, X.; Su, S.; Yan, H.; Guo, S.; Qian, D.; Duan, J.A. Investigation of dynamic accumulation and regularity of nine glycosides and saccharides in Rehmannia glutinosa by rapid quantitative analysis technology. J. Sep. Sci. 2019, 42, 1489–1499. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, G.; Ma, S.; Li, F.; Yuan, M.; Xu, H.; Huang, K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-kappaB pathways. Biochem. Biophys. Res. Commun. 2015, 467, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Deng, H.; Zhang, Q.; Xie, J.; Zeng, H.; Jin, X.; Ling, Z.; Shan, Q.; Liu, M.; Ma, Y.; et al. Amelioration of Diabetic Mouse Nephropathy by Catalpol Correlates with Down-Regulation of Grb10 Expression and Activation of Insulin-Like Growth Factor 1 / Insulin-Like Growth Factor 1 Receptor Signaling. PLoS ONE 2016, 11, e0151857. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, L.; Li, X.; Jiang, Z.; Sun, L.; Zhao, G.; Zhou, G.; Zhang, H.; Shang, J.; Wang, T. Mitochondrial fusion/fission process involved in the improvement of catalpol on high glucose-induced hepatic mitochondrial dysfunction. Acta Biochim. Et Biophys. Sin. 2015, 47, 730–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, Z.; Jiang, Z.; Sun, L.; Ji, J.; Miao, J.; Zhang, X.; Li, X.; Huang, S.; Wang, T.; et al. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochim. Et Biophys. Sin. 2014, 46, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, G.; Zhong, W.; Wu, F.; Wang, X.; Liu, L. Catalpol attenuates cardiomyocyte apoptosis in diabetic cardiomyopathy via Neat1/miR-140-5p/HDAC4 axis. Biochimie 2019, 2019, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, L.; Jiang, Z.; Zhao, G.; Hassan, H.M.; Sun, L.; Fan, S.; Zhou, Z.; Zhang, L.; Wang, T. A new hypoglycemic mechanism of catalpol revealed by enhancing MyoD/MyoG-mediated myogenesis. Life Sci. 2018, 209, 313–323. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.R.; Hou, Y.B.; Jing, X.L.; Song, X.Y.; Shen, X.P. Global gene expression analysis in liver of db/db mice treated with catalpol. Chin. J. Nat. Med. 2018, 16, 590–598. [Google Scholar] [CrossRef]
- Bao, Q.; Shen, X.; Qian, L.; Gong, C.; Nie, M.; Dong, Y. Anti-diabetic activities of catalpol in db/db mice. Korean J. Physiol. Pharmacol. 2016, 20, 153–160. [Google Scholar] [CrossRef]
- Zhao, S.R.; Lu, Y.W.; Chen, J.L.; Duan, H.F.; Wu, Z.Z. Experimental Study on the Hypoglycemic Activity of Catalpol from Rehmannia glutinosa Libosch. LiShizhen Med. Mater. Med. Res. 2009, 20, 171–172. [Google Scholar]
- Wang, W.; Jia, C.; Pang, X.C.; Li, X.N. Comparative Study on Therapeutic Effect of Catalpol, Berberine and Their Compatibility on KK-Ay Diabetes Mellitus Mice. Res. Pract. Chin. Med. 2016, 30, 25–30. [Google Scholar]
- Huang, W.J.; Niu, H.S.; Lin, M.H.; Cheng, J.T.; Hsu, F.L. Antihyperglycemic effect of catalpol in streptozotocin-induced diabetic rats. J. Nat. Prod. 2010, 73, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.L.; Zhong, W.L.; Xu, R.; Shi, C.K.; Zhao, S.; Han, Y.B.; Liu, L. The Protective Effects of Catalpol on the Islet Cells in Rats with Type 2 Diabetes Mellitus. Chin. J. Integr. Med. Cardio-/Cerebrovasc. Dis. 2016, 14, 1727–1729. [Google Scholar]
- Zou, G.L.; Zhong, W.L.; Shi, C.K.; Xu, R.; Zhao, S.; Liu, L. The influence of catalpol on the expression of insulin in islet cells of diabetic rats. Chin. J. Integr. Med. Cardio-/Cerebrovasc. Dis. 2018, 16, 865–867. [Google Scholar]
- Zhu, H.; Wang, Y.; Liu, Z.; Wang, J.; Wan, D.; Feng, S.; Yang, X.; Wang, T. Antidiabetic and antioxidant effects of catalpol extracted from Rehmannia glutinosa (Di Huang) on rat diabetes induced by streptozotocin and high-fat, high-sugar feed. Chin. Med. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Shieh, J.P.; Cheng, K.C.; Chung, H.H.; Kerh, Y.F.; Yeh, C.H.; Cheng, J.T. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2011, 59, 3747–3753. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Zou, G.L. Effect of Catalpa on cardiac function and cardiac tissue of diabetic cardiomyopathy rats. Jilin J. Chin. Med. 2018, 38, 573–575. [Google Scholar]
- Jiang, P.; Xiang, L.; Chen, Z.; Lu, H.; Zhou, L.; Yang, L.; Ji, Y.; Liu, Y.; Sun, X.; Deng, Y.; et al. Catalpol alleviates renal damage by improving lipid metabolism in diabetic db/db mice. Am. J. Transl. Res. 2018, 10, 1750–1761. [Google Scholar]
- Zhao, J. Effection of Catapol on the Expression of IGF-1 and Akt in Kidney of Diabetic Rats. Master’s Thesis, Dalian Medical University, Liaoning, China, 2009. [Google Scholar]
- Liu, J.Y.; Zheng, C.Z.; Hao, X.P.; Zhang, D.J.; Mao, A.W.; Yuan, P. Catalpol ameliorates diabetic atherosclerosis in diabetic rabbits. Am. J. Transl. Res. 2016, 8, 4278–4288. [Google Scholar]
- Liu, J.Y. Catalpol protect diabetic vascular endothelial function by inhibiting NADPH oxidase. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2014, 39, 2936–2941. [Google Scholar]
- Zhou, H.; Liu, J.; Ren, L.; Liu, W.; Xing, Q.; Men, L.; Song, G.; Du, J. Relationship between [corrected] spatial memory in diabetic rats and protein kinase Cgamma, caveolin-1 in the hippocampus and neuroprotective effect of catalpol. Chin. Med. J. 2014, 127, 916–923. [Google Scholar] [PubMed]
- Liu, D. Study on the Effects of Catalpol and the Mechanism in Experimental Diabetic Peripheral Neuropathy. Master’s Thesis, Dalian Medical University, Liaoning, China, 2006. [Google Scholar]
- Zhou, H.C. Effection of Catalpol on the Expression of IGF-1 and Akt in Dorsal Root Ganglia and Sciatic Nerve of Diabetic Rats. Master’s Thesis, Dalian Medical University, Liaoning, China, 2007. [Google Scholar]
- Wang, C.F.; Li, D.Q.; Xue, H.Y.; Hu, B. Oral supplementation of catalpol ameliorates diabetic encephalopathy in rats. Brain Res. 2010, 10, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Chen, L.; Wang, X.Q.; Ku, B.Q. Effect of Catalpol on Insulin Resistance of Human Liver Cell HL-7702 and Its Mechanism. Tradit. Chin. Drug Res. Clin. Pharmacol. 2015, 26, 335–338. [Google Scholar]
- Zhou, X.; Liu, W.; Gu, M.; Zhou, H.; Zhang, G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J. Gastroenterol. 2015, 50, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Llopis, S.; Banuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, P.; Zhang, L.; Xiong, B.; Tao, J.; Guan, W.; Li, C.; Chen, C.; Gu, J.; Duanmu, J.; et al. Autophagy inhibition attenuates the induction of anti-inflammatory effect of catalpol in liver fibrosis. Biomed. Pharmacother. 2018, 103, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, Y.M.; Lee, M.S. The Role of Autophagy in Systemic Metabolism and Human-Type Diabetes. Mol. Cells 2018, 41, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Nolan, C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Matschinsky, F.M.; Madiraju, S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic Î2-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mugabo, Y.; Ballentine, G.; Attane, C.; Iglesias, J.; Poursharifi, P.; Zhang, D.; Nguyen, T.A.; Erb, H.; Prentki, R.; et al. alpha/beta-Hydrolase Domain 6 Deletion Induces Adipose Browning and Prevents Obesity and Type 2 Diabetes. Cell Rep. 2016, 14, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Madiraju, S.R. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol. Cell. Endocrinol. 2012, 353, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. BBA-Mol. Basis Dis. 2014, 1842, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Jang, H.J.; Chung, T.W.; Jeong, S.I.; Cha, J.; Choi, J.Y.; Han, C.W.; Jang, Y.S.; Joo, M.; Jeong, H.S.; et al. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia 2013, 86, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.N. Effect of Catalpol, Berberine and Their Combination on the Secretion and mRNA Expression of TNF-α, IL-1β and IL-6 in Insulin Resistant Adipocytes. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2010. [Google Scholar]
- Liu, M. Effect of Catalpol, Berberine and Their Combination on the Secretion and Expression of Resistin in Insulin Resistant 3T3-L1 Adipocytes. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2010. [Google Scholar]
- Wang, Z.Y.; Yang, M.W.; Chen, L.; Liu, Y.J.; Lu, F.E.; Huang, G.Y. Effect of Berberine, Catalpol and Their Combination on the Expression of Glut4, IRS-1, IRS-1Ser307 Phosphorylation in Insulin Resistant 3T3-L1 Adipocytes. China Pharm. 2008, 1142–1145. [Google Scholar] [CrossRef]
- Liu, F.F.; Yang, M.W.; Wang, X.Q.; Wang, K.F.; Lu, F.E. Effect of catalpol, berberine and their combination on insulin resistant 3T3-L1 adipocytes. Chin. Tradit. Herb. Drugs 2007, 1523–1526. [Google Scholar]
- Liu, F.F. Research on the Effect of Berberine, Catalpol and Their Combination Relieving IR. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2007. [Google Scholar]
- Li, C.; Mingwei, Y.; Zhongyu, W.; Yanjuan, L.; Fuer, L.; Guangying, H. Effect of catalpol, berberine, and their combination on expression of Glut4 protein and C-Cb1 associated protein in insulin resistant 3T3-L1 adipocytes. Chin. Tradit. Herb. Drugs 2008, 39, 1510–1514. [Google Scholar]
- Fujimaki, S.; Kuwabara, T. Diabetes-Induced Dysfunction of Mitochondria and Stem Cells in Skeletal Muscle and the Nervous System. Int. J. Mol. Sci. 2017, 18, 2147. [Google Scholar] [CrossRef]
- Barlow, J.P.; Solomon, T.P. Do skeletal muscle-secreted factors influence the function of pancreatic beta-cells? Am. J. Physiol. Endocrinol. Metab. 2018, 314, E297–E307. [Google Scholar] [CrossRef]
- Halling, J.F.; Jessen, H.; Nohr-Meldgaard, J.; Thiellesen Buch, B.; Masselkhi Christensen, N.; Gudiksen, A.; Ringholm, S.; Neufer, P.D.; Prats, C.; Pilegaard, H. PGC-1alpha regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am. J. Physiol. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- Galon-Tilleman, H.; Yang, H.; Bednarek, M.A.; Spurlock, S.M.; Paavola, K.J.; Ko, B.; To, C.; Luo, J.; Tian, H.; Jermutus, L.; et al. Apelin-36 Modulates Blood Glucose and Body Weight Independently of Canonical APJ Receptor Signaling. J. Biol. Chem. 2017, 292, 1925–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, W.J.; Tang, L.Q.; Wei, W. Research progress in signalling pathway in diabetic nephropathy. Diabetes/Metab. Res. Rev. 2015, 31, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; Boer, I.H.D.; Goldsteinfuchs, J.; Hirsch, I.B.; Kalantarzadeh, K.; Narva, A.S.; Navaneethan, S.D. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, M.K.; Singh, U.K. Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update. Vasc. Pharmacol. 2013, 58, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.L.; Shen, Y.; Shao, D.C.; Miao, N.J.; Zhou, J.L. Insulin deficiency induces rat renal mesangial cell dysfunction via activation of IGF-1/IGF-1R pathway. Acta Pharmacol. Sin. 2016, 37, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, T.T.; Chen, J.; Qiu, W. Elevated expression levels of serum insulin-like growth factor-1, tumor necrosis factor-α and vascular endothelial growth factor 165 might exacerbate type 2 diabetic nephropathy. J. Diabetes Investig. 2017, 8, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sandro, G.; Bonventre, J.V.; Kyriakis, J.M. Signaling pathways and late-onset gene induction associated with renal mesangial cell hypertrophy. Embo J. 2014, 21, 5427–5436. [Google Scholar]
- Ivan, T.; Elliot, S.J.; Mylene, P.; Ana, R.; Striker, G.E.; Striker, L.J. Autocrine activation of the IGF-I signaling pathway in mesangial cells isolated from diabetic NOD mice. Diabetes 2002, 51, 182–188. [Google Scholar]
- Zhang, Y.; Jiong, X.U.; Yang, W.H. Correlative evaluation on serum IGF-1 and TNF-α of Type 2 diabetes patients. J. Tianjin Med. Univ. 2004, 2, 288–289, 315. [Google Scholar] [CrossRef]
- Tschöpe, C.; Schultheiss, H.P. Diabetic cardiopathy: Pathogenesis, diagnosis and therapy. Der Internist 2003, 44, 814–818. [Google Scholar]
- Vadla, G.P.; Vellaichamy, E. RETRACTED: Anti-fibrotic cardio protective efficacy of aminoguanidine against streptozotocin induced cardiac fibrosis and high glucose induced collagen up regulation in cardiac fibroblasts. Chem.-Biol. Interact. 2012, 197, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, R.; Overstreet, J.M.; Higgins, P.J. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell. Signal. 2013, 25, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.F.; Li, Y.L.; Jia, L.X.; Han, Y.L.; Cheng, J.Z.; Li, H.H.; Qi, Y.F.; Du, J. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF β/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE 2012, 7, e35144. [Google Scholar] [CrossRef]
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S103–S123. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Siddiqui, Z.; Rehman, S.; Khan, M.Y.; Khan, H.; Khanum, S.; Alouffi, S.; Saeed, M. A Glycation Angle to Look into the Diabetic Vasculopathy: Cause and Cure. Curr. Vasc. Pharmacol. 2017, 15, 352–364. [Google Scholar] [CrossRef]
- Li, Z.Z.; Sun, S.W.; Nie, J.; Xu, Y.; Liang, X. Study on protective effects of catalpol on HUVECs injured by high glucose. Pharmacol. Clin. Chin. Mater. Med. 2017, 33, 27–31. [Google Scholar]
- Dong, Q.; Sun, L.; Hu, X.M.; Chen, J.; Zhou, F.; Huang, Q.; Xu, Y.C. Effects of Catalpol on the Inflammatory Factors of Endothelial Cells. Med. J. Wuhan Univ. 2016, 37, 884–887. [Google Scholar]
- Hong, H.; Fu, S.P.; Ou, C.; Zhu, B.M. Protective Effect of Catalpol on Vascular Endothelial Cell Injured by High Glucose. Mod. Tradit. Chin. Med. Mater. Med. -World Sci. Technol. 2015, 17, 846–849. [Google Scholar]
- Bi, F.; Xu, Y.; Sun, Q. Catalpol pretreatment attenuates cardiac dysfunction following myocardial infarction in rats. Anatol. J. Cardiol. 2018, 19, 296–302. [Google Scholar] [CrossRef]
- Huang, C.L.; Cui, Y.L.; Ji, L.L.; Zhang, W.; Li, R.; Ma, L.; Xing, W.J.; Zhou, H.P.; Chen, B.Y.; Yu, J. Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm. Biol. 2013, 51, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Santaniello, E. Peroxynitrite and nitrosoperoxycarbonate, a tightly connected oxidizing-nitrating couple in the reactive nitrogen-oxygen species family: New perspectives for protection from radical-promoted injury by flavonoids. J. Pharm. Pharmacol. 2007, 59, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Yasin Wayhs, C.A.; Tannhauser Barros, H.M.; Vargas, C.R. GABAergic Modulation in Diabetic Encephalopathy-Related Depression. Curr. Pharm. Des. 2015, 21, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.S.; Duarte, C.B.; Faro, C.J.; Pires, E.V.; Carvalho, A.L. Protein kinase C gamma associates directly with the GluR4 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. Effect on receptor phosphorylation. J. Biol. Chem. 2003, 278, 6307–6313. [Google Scholar] [CrossRef] [PubMed]
- Baydas, G.; Donder, E.; Kiliboz, M.; Sonkaya, E.; Tuzcu, M.; Yasar, A.; Nedzvetskii, V.S. Neuroprotection by alpha-lipoic acid in streptozotocin-induced diabetes. Biochemistry (Moscow) 2004, 69, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Brixius, K.; Brinkmann, C. Exercise for the diabetic brain: How physical training may help prevent dementia and Alzheimer’s disease in T2DM patients. Endocrine 2016, 53, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Z.; Wu, J.; Xiang, S.; Sheng, S.; Jiang, Y.; Yang, Z.; Hua, F. Catalpol preserves neural function and attenuates the pathology of Alzheimer’s disease in mice. Mol. Med. Rep. 2016, 13, 491–496. [Google Scholar] [CrossRef]
- Wang, J.H.; Xie, H.; Zhao, T.K.; Kang, B. Catalpol regulates cholinergic nerve system function through effect on choline acetyl-transferase not M receptor affinity. Biomed. Pharmacother. 2015, 69, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, F.; Zhou, S.; Zhang, R.; Wang, F.; Huang, J.H.; Wu, E.; Zhang, Y.; Hu, Y. Catalpol protects synaptic proteins from beta-amyloid induced neuron injury and improves cognitive functions in aged rats. Oncotarget 2017, 8, 69303–69315. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Li, W.T.; Yu, S.T.; Xie, H.; Han, H.R. Catalpol regulates function of hypothalamic-pituitary-adrenocortical-axis in an Alzheimer’s disease rat model. Die Pharm. 2014, 69, 688–693. [Google Scholar]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef]
- Ma, R.; Zhu, R.; Wang, L.; Guo, Y.; Liu, C.; Liu, H.; Liu, F.; Li, H.; Li, Y.; Fu, M.; et al. Diabetic Osteoporosis: A Review of Its Traditional Chinese Medicinal Use and Clinical and Preclinical Research. Evid.-Based Complement. Altern. Med. 2016, 2016, 3218313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.D. Studies on Chemical Constituents and Mechanism of Rehmanniae Radix Preparata for Diabetic Osteoporosis Based on Molecular Docking Strategy. Master’s Thesis, Second Military Medical University, Shanghai, China, 2016. [Google Scholar]
- Lai, N.; Zhang, J.; Ma, X.; Wang, B.; Miao, X.; Wang, Z.; Guo, Y.; Wang, L.; Yao, C.; Li, X.; et al. Regulatory Effect of Catalpol on Th1/Th2 cells in Mice with Bone Loss Induced by Estrogen Deficiency. Am. J. Reprod. Immunol. 2015, 74, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I. Interaction between bone and glucose metabolism [Review]. Endocr. J. 2017, 64, 1043–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Gu, Y.; Si, D.; Liu, C. Quantitation of catalpol in rat plasma by liquid chromatography/electrospray ionization tandem mass spectrometry and its pharmacokinetic study. J. Chromatogr. B 2009, 877, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.; Lu, R.; Gu, Y.; Liu, C.X. Pharmacokinetics and bioavalibability of catalpol in rats. Chin. J. Clin. Pharmacol. Ther. 2012, 17, 126–130. [Google Scholar]
- Wang, Q.; Xing, M.; Chen, W.; Zhang, J.; Qi, H.; Xu, X. HPLC–APCI–MS/MS method for the determination of catalpol in rat plasma and cerebrospinal fluid: Application to an in vivo pharmacokinetic study. J. Pharm Biomed. Anal. 2012, 70, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.K.; Liu, Z.Z.; Peng, Y.; Zhang, L.H.; Ju, P.; Bi, K.S.; Chen, X.H. Validated LC-MS method for simultaneous quantitation of catalpol and harpagide in rat plasma: Application to a comparative pharmacokinetic study in normal and diabetic rats after oral administration of Zeng-Ye-Decoction. Biomed. Chromatogr. 2013, 27, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qian, D.; Liu, P.; Shang, E.X.; Jiang, S.; Guo, J.; Su, S.L.; Duan, J.A.; Du, L.; Tao, J. Comparative pharmacokinetics of catalpol and acteoside in normal and chronic kidney disease rats after oral administration of Rehmannia glutinosa extract. Biomed. Chromatogr. BMC 2015, 29, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q. Pharmacokinetics of Catalpol after Different Administrations in Rats. Master’s Thesis, Southwestern University, Chongqing, China, 2014. [Google Scholar]
- Chen, S.Y.; Zhou, J.; Li, M.; Fan, H.J.; Zhang, Y.Y. Metabolic Change of Catalpol in Rehmannia Glutinosa Libosch in Rats. J. Emerg. Tradit. Chin. Med. 2018, 27, 1197–1200. [Google Scholar]
- Dong, W.; Jing, X.U.; Chen, L. Separation and Identification of Catalpol from Rehmannia and its Acute Toxicity Test. J. Fudan Univ. 2009, 48, 409–412. [Google Scholar]
- Jiang, T.; Zhang, A.; Zhao, R.; Jiang, B. Protective Effect of Catalpol in Mice Injuries Induced by Rotenone and Evaluation of the Safety of Catalpol. Prog. Mod. Biomed. 2008, 8, 1039–1041, 1045. [Google Scholar] [CrossRef]
- Fei, B.; Dai, W.; Zhao, S. Efficacy, Safety, and Cost of Therapy of the Traditional Chinese Medicine, Catalpol, in Patients Following Surgical Resection for Locally Advanced Colon Cancer. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2018, 24, 3184–3192. [Google Scholar] [CrossRef] [PubMed]






| Animal Model | Route of Administration | Dose of Catalpol | Treatment Duration | Reference |
|---|---|---|---|---|
| C57BL/6J + HFD | oral | 100 mg/kg | 4w | [22] |
| C57BL6/J + HFD/STZ (40 mg/kg) | oral | 100, 200 mg/kg | 4w | [23] |
| C57BL/6 + STZ (180 mg/kg) | i.p. | 10 mg/kg | 2w | [24] |
| C57BL/6J + HFD/STZ (85 mg/kg) | oral | 50, 100, 200 mg/kg | 4w | [25,26] |
| C57BL6/J + HFHG/STZ (100 mg/kg) | oral | 10 mg/kg | 12w | [27] |
| db/db mice | oral | 200 mg/kg | 8w | [28] |
| db/db mice | oral | 20, 50, 100, 200 mg/kg | 8w | [29] |
| db/db mice | oral | 40, 80, 120 mg/kg | 4w | [30] |
| KM + ALX (60 mg/kg) | oral | 50, 100, 200 mg/kg | 2w | [31] |
| KK-Ay mice | oral | 200 mg/kg | 8w | [32] |
| Wistar + STZ (60 mg/kg) | i.v. | 0.1 mg/kg | / | [33] |
| Wistar + STZ (15 mg/kg) | oral | 2.5, 5, 10 mg/kg | 12w | [34,35] |
| Wistar + STZ (30 mg/kg) | i.v. | 5, 10, 20, 50 mg/kg | 2w + 2w | [36] |
| Wistar + STZ (65 mg/kg) BDF1/opioid receptor gene knockout mice + STZ (50 mg/kg) | i.v. | 0.1 mg/kg | 3d | [37] |
| Wistar + STZ (15 mg/kg) | oral | 2.5, 5, 10 mg/kg | 12w | [38] |
| db/db mice | chow diet supplemented with catalpol | 1 g/kg | 16w | [39] |
| SD + STZ (50 mg/kg) | i.p. | 5 mg/kg | 2w | [40] |
| Rabbit + ALX (100 mg/kg) | oral | 5 mg/kg | 12w | [41] |
| Wistar + STZ (30 mg/kg) | oral | 10, 50, 100 mg/kg | 6w | [42] |
| SD + STZ (50 mg/kg) | i.p. | 5 mg/kg | 2w | [43,44,45] |
| SD + STZ (65 mg/kg) | oral | 10, 50, 100 mg/kg | 6w | [46] |
| Experimental Animal | Dosage and Methods | Pharmacokinetic Parameters | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t1/2/h | AUC/(mg·h·L−1) | MRT/h | Cmax/(mg/L) | Tmax/h | Vz/F/(L·kg−1) | F% | |||
| Healthy male SD rats | 50 mg/kg, gavage | 1.39 ± 0.22 | 95.23 ± 10.15 | 3.23 ± 0.37 | 24.83 ± 0.58 | 1.66 ± 0.58 | 1.17 ± 0.16 | 49.38 ± 10.54 | [18] |
| Healthy male SD rats | 50 mg/kg, i.p. | 0.84 ± 0.41 | 150.23 ± 20.87 | 1.62 ± 0.20 | 80.43 ± 5.59 | 0.25 ± 0.1 | 0.40 ± 0.20 | 71.62 ± 10.28 | [18] |
| Healthy male SD rats | 50 mg/kg, i.v. | 0.68 ± 0.24 | 195.79 ± 20.51 | 1.71 ± 0.29 | 110.82 ± 4.10 | / | 0.57 ± 0.19 | / | [18] |
| Healthy male and female Wistar rats | 50 mg/kg, gavage | 1.21 ± 0.39 | 69.52 ± 22.93 | 3.27 ± 0.37 | 23.32 ± 10.47 | 1.33 ± 0.41 | 1.43 ± 0.68 | / | [102] |
| Healthy male and female Wistar rats | 50 mg/kg, gavage | 1.8 ± 1.3 | 70 ± 23 | 3.3 ± 0.4 | 23 ± 10 | 1.3 ± 0.4 | 3.6 ± 1.4 | 66.70 | [103] |
| Healthy male and female Wistar rats | 100 mg/kg, gavage | 1.7 ± 1.0 | 118 ± 27 | 3.4 ± 0.5 | 35 ± 14 | 36 ± 9 | 3.7 ± 3.1 | / | [103] |
| Healthy male and female Wistar rats | 200 mg/kg, gavage | 2.6 ± 1.5 | 217 ± 49 | 6.8 ± 1.2 | 36 ± 9 | 2.3 ± 0.8 | 14.7±8.8 | / | [103] |
| Healthy male and female Wistar rats | 50 mg/kg, i.v. | 1.9 ± 1.5 | 104 ± 11 | 1.9 ± 0.4 | / | / | 4.1 ± 2.0 | / | [103] |
| Healthy male SD rats | 6 mg/kg, i.v. (in CSF) | 1.52 ± 0.74 | 0.67 ± 0.11 | 2.12 ± 1.0 | 0.68 ± 0.20 | / | / | / | [104] |
| Healthy male SD rats | 6 mg/kg, i.v. (in plasma) | 0.71 ± 0.23 | 11.53 ± 1.64 | 0.70 ± 0.20 | 23.61 ± 0.91 | / | / | / | [104] |
| Healthy male SD rats | 37.2 mg/kg, gavage | 4.0 ± 1.0 | 93.7 ± 38.6 | / | 11.82 ± 6.79 | 3.1 ± 1.7 | / | / | [105] |
| STZ (55 mg/kg) induced diabetic rats | 37.3 mg/kg, gavage | 11.6 ± 4.2 | 220.2 ± 79.6 | / | 37.41 ± 13.01 | 2.4 ± 1.1 | / | / | [106] |
| Healthy male SD rats | 8.0 mg/kg, gavage | 1.4 ± 0.2 | 7.41 ± 0.68 | / | 2.14 ± 0.13 | 1.01 ± 0.25 | / | / | [106] |
| Doxorubicin (5.0 mg/kg) induced CKD rats | 8.0 mg/kg, gavage | 2.0 ± 0.7 | 27.64 ± 4.20 | / | 7.94 ± 1.06 | 0.90 ± 0.14 | / | / | [106] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Zhu, R.; Tian, Y.; Li, R.; Chen, B.; Zhang, H.; Xia, B.; Zhao, D.; Mo, F.; Zhang, D.; et al. Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety. Molecules 2019, 24, 3302. https://doi.org/10.3390/molecules24183302
Bai Y, Zhu R, Tian Y, Li R, Chen B, Zhang H, Xia B, Zhao D, Mo F, Zhang D, et al. Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety. Molecules. 2019; 24(18):3302. https://doi.org/10.3390/molecules24183302
Chicago/Turabian StyleBai, Ying, Ruyuan Zhu, Yimiao Tian, Rui Li, Beibei Chen, Hao Zhang, Bingke Xia, Dandan Zhao, Fangfang Mo, Dongwei Zhang, and et al. 2019. "Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety" Molecules 24, no. 18: 3302. https://doi.org/10.3390/molecules24183302