Investigation of the Phase Transition Mechanism in LiFePO4 Cathode Using In Situ Raman Spectroscopy and 2D Correlation Spectroscopy during Initial Cycle
Abstract
:1. Introduction
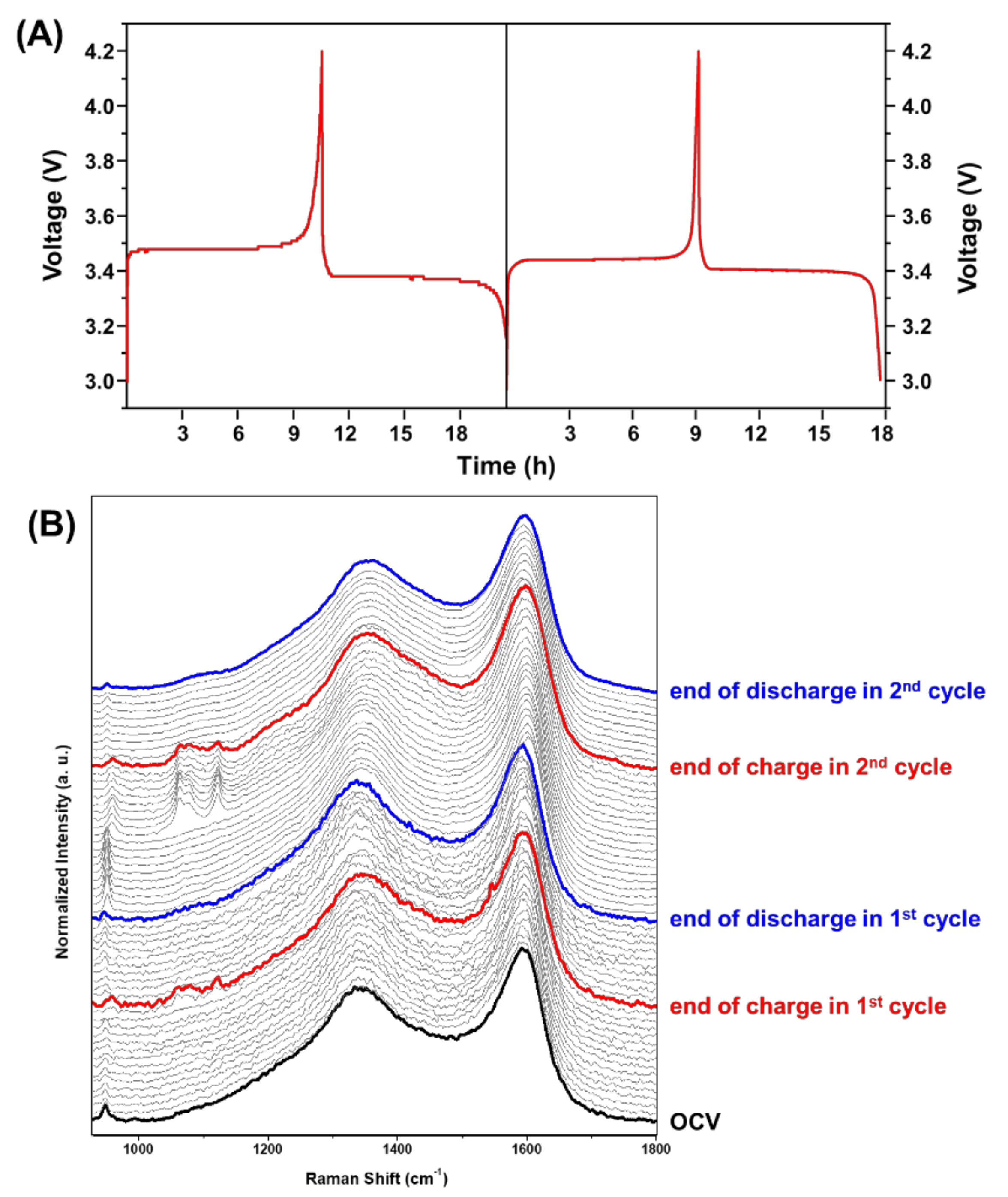
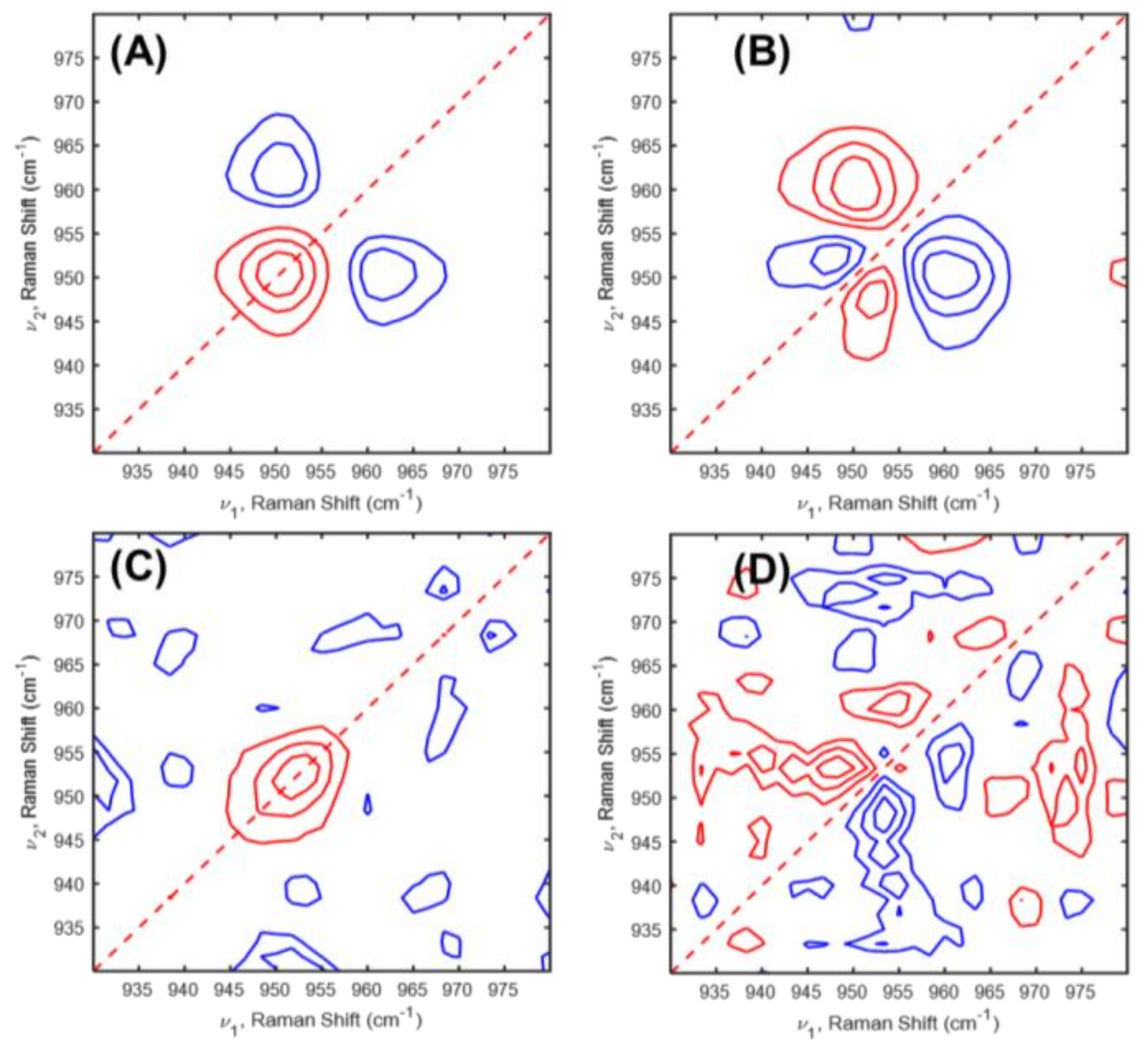
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.-J.; Jaye, C.; Nam, K.-W.; Zhang, B.; Chen, H.-Y.; Bai, J.; Li, H.; Huang, X.; Fischer, D.A.; Yang, X.-Q. Investigation of the structural changes in Li1−xFePO4 upon charging by synchrotron radiation techniques. J. Mater. Chem. 2011, 21, 11406–11411. [Google Scholar] [CrossRef]
- Dong, H.; Guo, H.; He, Y.; Gao, J.; Han, W.; Lu, X.; Yan, S.; Yang, K.; Li, H.; Chen, D. Structural stability and Li-ion transport property of LiFePO4 under high-pressure. Solid State Ion. 2017, 301, 133–137. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Huang, J.; Qu, Y.; Niu, C.; Du, Z.; Li, D.; Chen, Y. Coin-Cell-Based In Situ Characterization Techniques for Li-Ion Batteries. Front. Energy Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Siddique, N.A.; Salehi, A.; Wei, Z.; Liu, D.; Sajjad, S.D.; Liu, F. Length-Scale-Dependent Phase Transformation of LiFePO4: An In situ and Operando Study Using Micro-Raman Spectroscopy and XRD. ChemPhysChem 2015, 16, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Ruther, R.E.; Zhou, H.; Dhital, C.; Saravanan, K.; Kercher, A.K.; Chen, G.; Huq, A.; Delnick, F.M.; Nanda, J. Synthesis, Structure, and Electrochemical Performance of High Capacity Li2Cu0.5Ni0.5O2 Cathodes. Chem. Mater. 2015, 27, 6746–6754. [Google Scholar] [CrossRef]
- Li, B.; Shao, R.; Yan, H.; An, L.; Zhang, B.; Wei, H.; Ma, J.; Xia, D.; Han, X. Understanding the Stability for Li-Rich Layered Oxide Li2RuO3 Cathode. Adv. Funct. Mater. 2016, 26, 1330–1337. [Google Scholar] [CrossRef]
- Kim, S.; Zhang, Z.; Wang, S.; Yang, L.; Cairns, E.J.; Penner-Hahn, J.E.; Deb, A. Electrochemical and Structural Investigation of the Mechanism of Irreversibility in Li3V2(PO4)3 Cathodes. J. Phys. Chem. C 2016, 120, 7005–7012. [Google Scholar] [CrossRef]
- Dalgleish, T.; Williams, J.M.G.; Golden, A.-M.J.; Perkins, N.; Barrett, L.F.; Barnard, P.J.; Au Yeung, C.; Murphy, V.; Elward, R.; Tchanturia, K. In-situ Raman Microscopy of Idividual LiNi0.8Co0.15Al0.05O2 Particles in the Li-ion Battery Composite Cathode. J. Exp. Psychol. Gen. 2007, 136, 23–42. [Google Scholar] [CrossRef]
- Fukumitsu, H.; Omori, M.; Terada, K.; Suehiro, S. Development of In Situ Cross-Sectional Raman Imaging of LiCoO2 Cathode for Li-ion Battery. Electrochemistry 2015, 83, 993–996. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Hahn, M.; Ruch, P.; Holzapfel, M.; Scheifele, W.; Buqa, H.; Krumeich, F.; Novák, P.; Kötz, R. An in situ Raman study of the intercalation of supercapacitor-type electrolyte into microcrystalline graphite. Electrochim. Acta 2006, 52, 675–680. [Google Scholar] [CrossRef]
- Hy, S.; Chen, Y.-H.; Liu, J.; Rick, J.; Hwang, B.-J. In situ surface enhanced Raman spectroscopic studies of solid electrolyte interphase formation in lithium ion battery electrodes. J. Power Sources 2014, 256, 324–328. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, D.; Trottier, J.; Gagnon, C.; Howe, J.; Mauger, A.; Julien, C.M.; Zaghib, K. In-situ Raman spectroscopic investigation of LiMn1.45Ni0.45M0.1O4 (M=Cr, Co) 5 V cathode materials. J. Power Sources 2015, 298, 341–348. [Google Scholar] [CrossRef]
- Wu, J.; Dathar, G.K.P.; Sun, C.; Theivanayagam, M.G.; Applestone, D.; Dylla, A.G.; Manthiram, A.; Henkelman, G.; Goodenough, J.B.; Stevenson, K.J. In situ Raman spectroscopy of LiFePO4: size and morphology dependence during charge and self-discharge. Nanotechnology 2013, 24, 424009. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Li, B.; Liu, B.; Liu, B.J.; Zhao, J.B.; Ren, B. Structural evolution of NM (Ni and Mn) lithium-rich layered material revealed by in-situ electrochemical Raman spectroscopic study. J. Power Sources 2016, 310, 85–90. [Google Scholar] [CrossRef]
- Stancovski, V.; Badilescu, S. In situ Raman spectroscopic-electrochemical studies of lithium-ion battery materials: A historical overview. J. Appl. Electrochem. 2014, 44, 23–43. [Google Scholar] [CrossRef]
- Ruch, P.W.; Hardwick, L.J.; Hahn, M.; Foelske, A.; Kötz, R.; Wokaun, A. Electrochemical doping of single-walled carbon nanotubes in double layer capacitors studied by in situ Raman spectroscopy. Carbon N. Y. 2009, 47, 38–52. [Google Scholar] [CrossRef]
- Gross, T.; Giebeler, L.; Hess, C. Novel in situ cell for Raman diagnostics of lithium-ion batteries. Rev. Sci. Instrum. 2013, 84, 073109/1–073109/7. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Goh, B.M.; Hu, M.Y.; Wan, C.; Tian, B.; Deng, X.; Peng, C.; Lin, M.; Hu, J.Z.; Loh, K.P. In Situ Raman and Nuclear Magnetic Resonance Study of Trapped Lithium in the Solid Electrolyte Interface of Reduced Graphene Oxide. J. Phys. Chem. C 2016, 120, 2600–2608. [Google Scholar] [CrossRef]
- Harks, P.P.R.M.L.; Mulder, F.M.; Notten, P.H.L. In situ methods for Li-ion battery research: A review of recent developments. J. Power Sources 2015, 288, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Seidl, L.; Martens, S.; Ma, J.; Stimming, U.; Schneider, O. In situ scanning tunneling microscopy studies of the SEI formation on graphite electrodes for Li+-ion batteries. Nanoscale 2016, 8, 14004–14014. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Li, X.; Wang, D.; Liu, J.; Liang, G.; Gauthier, M.; Li, Y.; Geng, D.; Li, R. Hierarchically porous LiFePO4/nitrogen-doped carbon nanotubes composite as a cathode for lithium ion batteries. J. Mater. Chem. 2012, 22, 7537–7543. [Google Scholar] [CrossRef]
- Nagpure, S.C.; Bhushan, B.; Babu, S.S. Raman and NMR studies of aged LiFePO4 cathode. Appl. Surf. Sci. 2012, 259, 49–54. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, T.; Liang, J.; Zhu, Y.; Zhang, K.; Qian, Y. Trace Fe3+ mediated synthesis of LiFePO4 micro/nanostructures towards improved electrochemical performance for lithium-ion batteries. RSC Adv. 2016, 6, 456–463. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C. Strain accommodation and potential hysteresis of LiFePO4 cathodes during lithium ion insertion/extraction. J. Power Sources 2011, 196, 1442–1448. [Google Scholar] [CrossRef]
- Yamanaka, T.; Abe, T.; Nishio, K.; Ogumi, Z. Diffusion of Li-deficient phases in large LiFePO4 single crystals during chemical delithiation. J. Mater. Chem. A 2018, 6, 11005–11011. [Google Scholar] [CrossRef]
- Yamada, A.; Koizumi, H.; Nishimura, S.; Sonoyama, N.; Kanno, R.; Yonemura, M.; Nakamura, T.; Kobayashi, Y. Room-temperature miscibility gap in LixFePO4. Nat. Mater. 2006, 5, 357–360. [Google Scholar] [CrossRef]
- Meethong, N.; Huang, H.-Y.S.; Carter, W.C.; Chiang, Y.-M. Size-Dependent Lithium Miscibility Gap in Nanoscale Li1-xFePO4. Electrochem. Solid-State Lett. 2007, 10, A134–A138. [Google Scholar] [CrossRef]
- Wagemaker, M.; Singh, D.P.; Borghols, W.J.H.; Lafont, U.; Haverkate, L.; Peterson, V.K.; Mulder, F.M. Dynamic Solubility Limits in Nanosized Olivine LiFePO4. J. Am. Chem. Soc. 2011, 133, 10222–10228. [Google Scholar] [CrossRef]
- Andersson, A.S.; Kalska, B.; Häggström, L.; Thomas, J.O. Lithium extraction/insertion in LiFePO4: An X-ray diffraction and Mössbauer spectroscopy study. Solid State Ion. 2000, 130, 41–52. [Google Scholar] [CrossRef]
- Srinivasan, V.; Newman, J. Discharge Model for the Lithium Iron-Phosphate Electrode. J. Electrochem. Soc. 2004, 151, A1517–A1529. [Google Scholar] [CrossRef]
- Delmas, C.; Maccario, M.; Croguennec, L.; Le Cras, F.; Weill, F. Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model. Nat. Mater. 2008, 7, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, Y.; Kim, S.M.; Jin, S.; Han, I.K.; Lee, S.M.; Jung, Y.M. Reaction at the Electrolyte–Electrode Interface in a Li-Ion Battery Studied by In Situ Raman Spectroscopy. Bull. Korean Chem. Soc. 2017, 38, 511–513. [Google Scholar] [CrossRef]
- Park, Y.; Shin, S.H.; Lee, S.M.; Kim, S.P.; Choi, H.C.; Jung, Y.M. 2D Raman correlation analysis of formation mechanism of passivating film on overcharged LiCoO2 electrode with additive system. J. Mol. Struct. 2014, 1069, 183–187. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Kim, J.Y.; Eom, I.-Y.; Jeong, Y.U.; Kim, M.S.; Lee, S.M.; Choi, H.C.; Jung, Y.M. Surface characterization of the high voltage LiCoO2/Li cell by X-ray photoelectron spectroscopy and 2D correlation analysis. Vib. Spectrosc. 2010, 53, 60–63. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Kim, J.M.; Kim, Y.C.; Jeong, Y.U.; Lee, S.M.; Choi, H.C.; Jung, Y.M. Surface Reaction of LiCoO2/Li System under High-Voltage Conditions by X-ray Spectroscopy and Two-Dimensional Correlation Spectroscopy (2D-COS). Appl. Spectrosc. 2011, 65, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, N.H.; Choi, H.C.; Lee, S.M.; Hwang, H.; Jeong, Y.U.; Jung, Y.M. Two-dimensional hetero-spectral Raman/XAS correlation analysis of Li[Ni0.45Co0.18Mn0.25Al0.12]O2 cathode in the overcharged state. Vib. Spectrosc. 2012, 60, 226–230. [Google Scholar] [CrossRef]
- Park, Y.; Shin, S.H.; Hwang, H.; Lee, S.M.; Kim, S.P.; Choi, H.C.; Jung, Y.M. Investigation of solid electrolyte interface (SEI) film on LiCoO2 cathode in fluoroethylene carbonate (FEC)-containing electrolyte by 2D correlation X-ray photoelectron spectroscopy (XPS). J. Mol. Struct. 2014, 1069, 157–163. [Google Scholar] [CrossRef]
- Choi, H.C.; Bin Kim, S.; Jung, Y.M. Application of soft X-ray absorption spectroscopy and two-dimensional correlation spectroscopy to the electrochemical reaction in the Li1+xV3O8/Li cell. J. Mol. Struct. 2006, 799, 91–95. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S. Bin Characterization of the Electrochemical Reactions in the Li1+xV3O8/Li Cell by Soft X-ray Absorption Spectroscopy and Two-Dimensional Correlation Analysis. Appl. Spectrosc. 2003, 57, 984–990. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Noda, I.; Kim, S. Bin A Study of the Mechanism of the Electrochemical Reaction of Lithium with CoO by Two-Dimensional Soft X-ray Absorption Spectroscopy (2D XAS), 2D Raman, and 2D Heterospectral XAS−Raman Correlation Analysis. J. Phys. Chem. B 2003, 107, 5806–5811. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Cho, S.B.; Kim, J.M.; Kim, G.C.; Kim, M.S.; Lee, S.M.; Eom, I.-Y.; Choi, H.C.; Jung, Y.M. Characterization of the passivating layer on Li[Ni0.31Co0.32Mn0.28Al0.09]O2 cathode in the overcharge state. J. Mol. Struct. 2010, 974, 139–143. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 1st ed.; Springer: New York, NY, USA, 1986. [Google Scholar]
- Martens, H.; Næs, T. Multivariate Calibration; John Wiley and Sons: New York, NY, USA, 1991. [Google Scholar]
- Vandeginste, B.G.M.; Massart, D.L. Handbook of Chemometrics and Qualimetrics: Part B; Elsevier Science B. V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Burba, C.M.; Palmer, J.M.; Holinsworth, B.S. Laser-induced phase changes in olivine FePO4: A warning on characterizing LiFePO4-based cathodes with Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 225–228. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Kim, S.M.; Jin, S.; Lee, S.M.; Noda, I.; Jung, Y.M. Investigation of the Phase Transition Mechanism in LiFePO4 Cathode Using In Situ Raman Spectroscopy and 2D Correlation Spectroscopy during Initial Cycle. Molecules 2019, 24, 291. https://doi.org/10.3390/molecules24020291
Park Y, Kim SM, Jin S, Lee SM, Noda I, Jung YM. Investigation of the Phase Transition Mechanism in LiFePO4 Cathode Using In Situ Raman Spectroscopy and 2D Correlation Spectroscopy during Initial Cycle. Molecules. 2019; 24(2):291. https://doi.org/10.3390/molecules24020291
Chicago/Turabian StylePark, Yeonju, Soo Min Kim, Sila Jin, Sung Man Lee, Isao Noda, and Young Mee Jung. 2019. "Investigation of the Phase Transition Mechanism in LiFePO4 Cathode Using In Situ Raman Spectroscopy and 2D Correlation Spectroscopy during Initial Cycle" Molecules 24, no. 2: 291. https://doi.org/10.3390/molecules24020291






