Protein Prenylation in Plant Stress Responses
Abstract
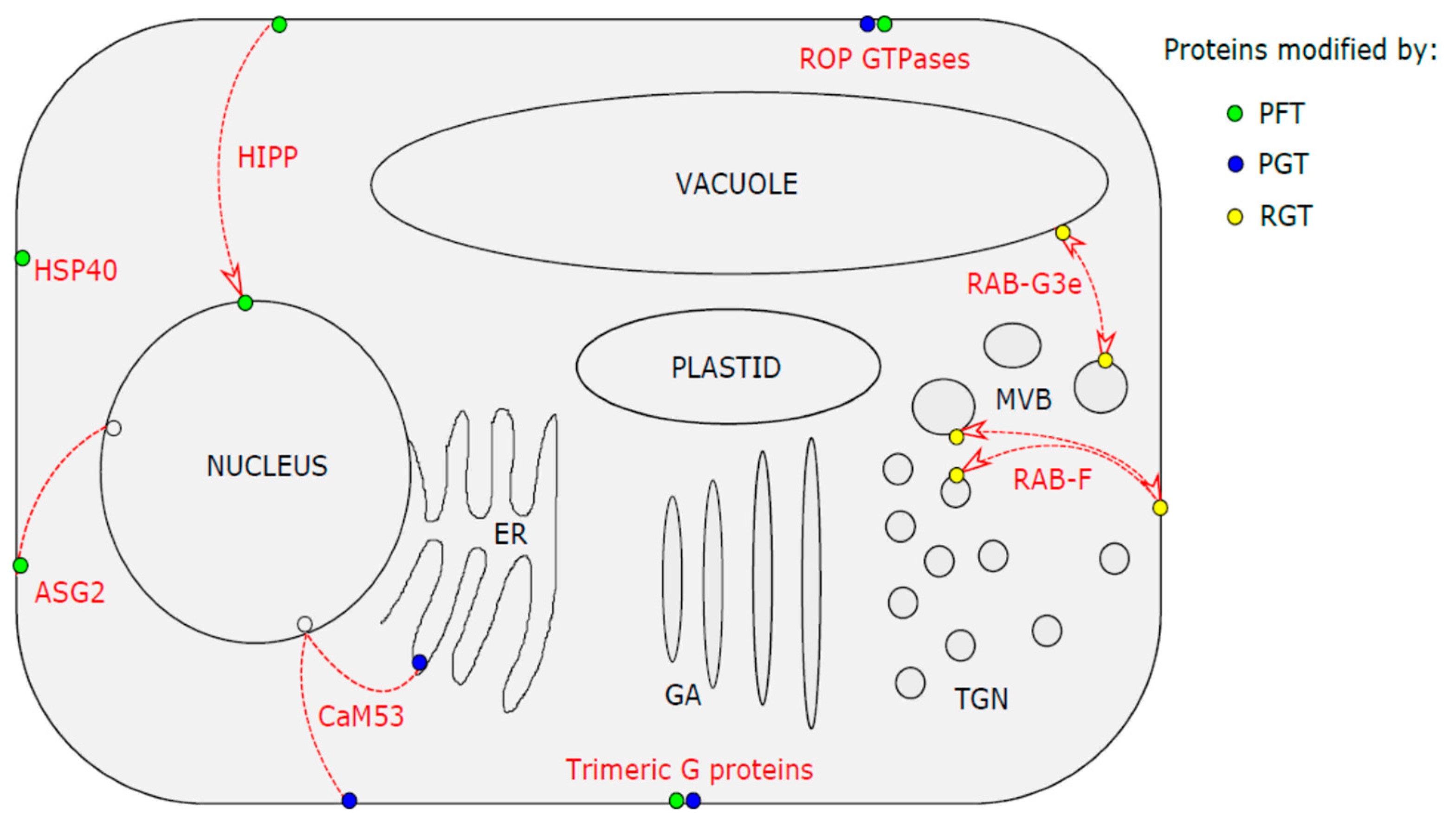
:1. Introduction to Protein Prenylation in Plants
1.1. Protein Farnesyl Transferase in Plant Stress Responses
1.2. Protein Geranylgeranyl Transferase I in Plant Stress Responses
1.3. Rab Geranylgeranyl Transferase
2. Substrates of Prenyl Transferases Involved in Abiotic Stress Responses
2.1. Heat Shock Protein HSP40
2.2. HIPP Proteins as Metallochaperons
2.2.1. AtFPs
2.2.2. HIPP26 and HIPP3
2.3. Heterotrimeric G- Proteins
2.4. Prenylated Calmodulin and Ca2+ Stress Signalling
2.5. Protein Altered Seed Germination 2 (ASG2)
2.6. ROP GTPases in Plant Stress Response
2.7. RAB GTPases in Plant Stress Response
3. Prenylated Proteins in Plant Biotic Stress Responses
4. Biotechnology Applications of Stress Prenylation Research in Plants
Funding
Conflicts of Interest
References
- Maurer-Stroh, S.; Washietl, S.; Eisenhaber, F. Protein prenyltransferases: anchor size, pseudogenes and parasites. Biol. Chem. 2003, 384, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Palsuledesai, C.C.; Distefano, M.D. Protein prenylation: enzymes, therapeutics, and biotechnology applications. ACS Chem Biol 2015, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D.; Hemsley, P.A. Fats and function: protein lipid modifications in plant cell signalling. Curr. Opin. Plant Biol. 2017, 40, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krute, C.N.; Carroll, R.K.; Rivera, F.E.; Weiss, A.; Young, R.M.; Shilling, A.; Botlani, M.; Varma, S.; Baker, B.J.; Shaw, L.N. The disruption of prenylation leads to pleiotropic rearrangements in cellular behavior in Staphylococcus aureus. Mol. Mar. 2015, 95, 819–832. [Google Scholar] [CrossRef]
- Suazo, K.F.; Schaber, C.; Palsuledesai, C.C.; Odom John, A.R.; Distefano, M.D. Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue. Sci Rep. 2016, 6, 38615. [Google Scholar] [CrossRef]
- Andrews, M.; Huizinga, D.H.; Crowell, D.N. The CaaX specificities of Arabidopsis protein prenyltransferases explain era1 and ggb phenotypes. BMC Plant Biol. 2010, 10, 118. [Google Scholar] [CrossRef]
- Desnoyers, L.; Seabra, M.C. Single prenyl-binding site on protein prenyl transferases. Proc. Natl. Acad. Sci. USA 1998, 95, 12266–12270. [Google Scholar] [CrossRef] [Green Version]
- Long, S.B.; Casey, P.J.; Beese, L.S. Reaction path of protein farnesyltransferase at atomic resolution. Nature 2002, 419, 645–650. [Google Scholar] [CrossRef]
- Trueblood, C.E.; Boyartchuk, V.L.; Picologlou, E.A.; Rozema, D.; Poulter, C.D.; Rine, J. The CaaX proteases, Afc1p and Rce1p, have overlapping but distinct substrate specificities. Mol. Cell Biol. 2000, 20, 4381–4392. [Google Scholar] [CrossRef]
- Bergo, M.O.; Leung, G.K.; Ambroziak, P.; Otto, J.C.; Casey, P.J.; Gomes, A.Q.; Seabra, M.C.; Young, S.G. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 2001, 276, 5841–5845. [Google Scholar] [CrossRef]
- Cadiñanos, J.; Varela, I.; Mandel, D.A.; Schmidt, W.K.; Díaz-Perales, A.; López-Otín, C.; Freije, J.M. AtFACE-2, a functional prenylated protein protease from Arabidopsis thaliana related to mammalian Ras-converting enzymes. J. Biol. Chem. 2003, 278, 42091–42097. [Google Scholar] [CrossRef] [PubMed]
- Bracha-Drori, K.; Shichrur, K.; Lubetzky, T.C.; Yalovsky, S. Functional analysis of Arabidopsis postprenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant. Physiol. 2008, 148, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, K.; Simon, I.; Yurchenko, V.; Iakovenko, A.; Rostkova, E.; Scheidig, A.J.; Goody, R.S. Characterization of the ternary complex between Rab7, REP-1 and Rab geranylgeranyl transferase. Eur. J. Biochem. 1999, 265, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Erdman, R.A.; Castellano, F.; Maltese, W.A. Prenylation of Rab8 GTPase by type I and type II geranylgeranyl transferases. Biochem. J. 1998, 333, 497–504. [Google Scholar] [CrossRef]
- Alexandrov, K.; Horiuchi, H.; Steele-Mortimer, O.; Seabra, M.C.; Zerial, M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated Rab proteins to their target membranes. EMBO J. 1994, 13, 5260–5273. [Google Scholar] [CrossRef]
- Hála, M.; Eliás, M.; Zárský, V. A specific feature of the angiosperm Rab escort protein (REP) and evolution of the REP/GDI superfamily. J. Mol. Biol. 2005, 348, 1299–1313. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Karamouzis, M.V.; Papavassiliou, A.G. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat. Rev. Drug Discov. 2007, 6, 541–555. [Google Scholar] [CrossRef]
- Gutkowska, M.; Swiezewska, E. Structure, regulation and cellular functions of Rab geranylgeranyl transferase and its cellular partner Rab Escort Protein. Mol. Membr. Biol. 2012, 29, 243–256. [Google Scholar] [CrossRef]
- Cutler, S.; Ghassemian, M.; Bonetta, D.; Cooney, S.; McCourt, P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 1996, 273, 1239–1241. [Google Scholar] [CrossRef]
- Bonetta, D.; Bayliss, P.; Sun, S.; Sage, T.; McCourt, P. Farnesylation is involved in meristem organization in Arabidopsis. Planta 2000, 211, 182–190. [Google Scholar] [CrossRef]
- Yalovsky, S.; Rodríguez-Concepción, M.; Bracha, K.; Toledo-Ortiz, G.; Gruissem, W. Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell 2000, 12, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.M.; Ghassemian, M.; Kwak, C.M.; McCourt, P.; Schroeder, J.I. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 1998, 282, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.J.; Murata, Y.; Chu, S.P.; Nafisi, M.; Schroeder, J.I. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell. 2002, 14, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Barghetti, A.; Sjögren, L.; Floris, M.; Paredes, E.B.; Wenkel, S.; Brodersen, P. Heat-shock protein 40 is the key farnesylation target in meristem size control, abscisic acid signaling, and drought resistance. Genes Dev. 2017, 31, 2282–2295. [Google Scholar] [CrossRef] [Green Version]
- Northey, J.G.; Liang, S.; Jamshed, M.; Deb, S.; Foo, E.; Reid, J.B.; McCourt, P.; Samuel, M.A. Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat. Plants 2016, 2, 16114. [Google Scholar] [CrossRef]
- Wu, J.R.; Wang, L.C.; Lin, Y.R.; Weng, C.P.; Yeh, C.H.; Wu, S.J. The Arabidopsis heat-intolerant 5 hit5)/enhanced response to aba 1 (era1) mutant reveals the crucial role of protein farnesylation in plant responses to heat stress. New Phytol. 2017, 213, 1181–1193. [Google Scholar] [CrossRef]
- Johnson, C.D.; Chary, S.N.; Chernoff, E.A.; Zeng, Q.; Running, M.P.; Crowell, D.N. Protein geranylgeranyltransferase I is involved in specific aspects of abscisic acid and auxin signaling in Arabidopsis. Plant Physiol. 2005, 139, 722–733. [Google Scholar] [CrossRef]
- Thole, J.M.; Perroud, P.F.; Quatrano, R.S.; Running, M.P. Prenylation is required for polar cell elongation, cell adhesion, and differentiation in Physcomitrella patens. Plant J. 2014, 78, 441–451. [Google Scholar] [CrossRef]
- Running, M.P.; Lavy, M.; Sternberg, H.; Galichet, A.; Gruissem, W.; Hake, S.; Ori, N.; Yalovsky, S. Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc. Natl. Acad. Sci. USA 2004, 101, 7815–7820. [Google Scholar] [CrossRef]
- Chai, S.; Ge, F.R.; Feng, Q.N.; Li, S.; Zhang, Y. PLURIPETALA mediates ROP2 localization and stability in parallel to SCN1 but synergistically with TIP1 in root hairs. Plant J. 2016, 86, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, M.; Swiezewski, S.; Sarnowski, T.J.; Plochocka, D.; Chelstowska, A.; Tolmachova, T.; Swiezewska, E. Cloning and characterization of Rab Escort Protein (REP) from Arabidopsis thaliana. Cell Biol. Int. 2007, 31, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hála, M.; Soukupová, H.; Synek, L.; Zárský, V. Arabidopsis RAB geranylgeranyl transferase beta-subunit mutant is constitutively photomorphogenic, and has shoot growth and gravitropic defects. Plant J. 2010, 62, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Gutkowska, M.; Wnuk, M.; Nowakowska, J.; Lichocka, M.; Stronkowski, M.M.; Swiezewska, E. Rab geranylgeranyl transferase β subunit is essential for male fertility and tip growth in Arabidopsis. J. Exp. Bot. 2015, 66, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Running, M.P. The role of lipid post-translational modification in plant developmental processes. Front. Plant Sci. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. Isoprenylation of the plant molecular chaperone ANJ1 facilitates membrane association and function at high temperature. Proc. Natl. Acad. Sci. USA 1993, 90, 8557–8561. [Google Scholar] [CrossRef]
- Dykema, P.E.; Sipes, P.R.; Marie, A.; Biermann, B.J.; Crowell, D.N.; Randall, S.K. A new class of proteins capable of binding transition metals. Plant Mol. Biol. 1999, 41, 139–150. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, S.; Li, H.Y.; Tsao, S.W.; Chye, M.L. Arabidopsis thaliana acyl-CoA-binding protein ACBP2 interacts with heavy-metal-binding farnesylated protein AtFP6. New Phytol. 2009, 181, 89–102. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant J. 2002, 32, 165–173. [Google Scholar] [CrossRef]
- Barth, O.; Zschiesche, W.; Siersleben, S.; Humbeck, K. Isolation of a novel barley cDNA encoding a nuclear protein involved in stress response and leaf senescence. Physiol. Plant 2004, 121, 282–293. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Zschiesche, W.; Barth, O.; Daniel, K.; Böhme, S.; Rausche, J.; Humbeck, K. The zinc-binding nuclear protein HIPP3 acts as an upstream regulator of the salicylate-dependent plant immunity pathway and of flowering time in Arabidopsis thaliana. New Phytol. 2015, 207, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Islam, S.M.; Tuteja, N. Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal. Behav. 2012, 7, 733–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urano, D.; Colaneri, A.; Jones, A.M. Gα modulates salt-induced cellular senescence and cell division in rice and maize. J. Exp. Bot. 2014, 65, 6553–6561. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Yalovsky, S.; Zik, M.; Fromm, H.; Gruissem, W. The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J. 1999, 18, 1996–2007. [Google Scholar] [CrossRef]
- Dutilleul, C.; Ribeiro, I.; Blanc, N.; Nezames, C.D.; Deng, X.W.; Zglobicki, P.; Palacio Barrera, A.M.; Atehortùa, L.; Courtois, M.; Labas, V.; et al. ASG2 is a farnesylated DWD protein that acts as ABA negative regulator in Arabidopsis. Plant Cell Environ. 2016, 39, 185–198. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, J.; Wang, J.; Xu, B.; Jin, Z. Overexpression of a Novel ROP Gene from the Banana (MaROP5g) Confers Increased Salt Stress Tolerance. Int J. Mol. Sci. 2018, 19, 3108. [Google Scholar] [CrossRef]
- Li, C.; Lu, H.; Li, W.; Yuan, M.; Fu, Y. A ROP2-RIC1 pathway fine-tunes mikrotubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ. 2017, 40, 1127–1142. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Nafisi, M.; Tam, A.; Li, H.; Crowell, D.N.; Chary, S.N.; Schroeder, J.I.; Shen, J.; Yang, Z. Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 2002, 14, 2787–2797. [Google Scholar] [CrossRef]
- Schultheiss, H.; Hensel, G.; Imani, J.; Broeders, S.; Sonnewald, U.; Kogel, K.H.; Kumlehn, J.; Hückelhoven, R. Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol. 2005, 139, 353–362. [Google Scholar] [CrossRef]
- Mazel, A.; Leshem, Y.; Tiwari, B.S.; Levine, A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 2004, 134, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ding, X.; Chang, T.; Wang, Z.; Liu, R.; Zeng, X.; Cai, Y.; Zhu, Y. Overexpression of a Vesicle Trafficking Gene, OsRab7, enhances salt tolerance in rice. Sci. World J. 2014, 2014, 483526. [Google Scholar] [CrossRef] [PubMed]
- Poraty-Gavra, L.; Zimmermann, P.; Haigis, S.; Bednarek, P.; Hazak, O.; Stelmakh, O.R.; Sadot, E.; Schulze-Lefert, P.; Gruissem, W.; Yalovsky, S. The Arabidopsis Rho of plants GTPase AtROP6 functions in developmental and pathogen response pathways. Plant Physiol. 2013, 161, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X. IAN/GIMAPs are conserved and novel regulators in vertebrates and angiosperm plants. Plant Signal. Behav 2009, 4, 165–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjögren, L.; Floris, M.; Barghetti, A.; Völlmy, F.; Linding, R.; Brodersen, P. Farnesylated heat shock protein 40 is a component of membrane-bound RISC in Arabidopsis. J. Biol. Chem. 2018, 293, 16608–16622. [Google Scholar] [CrossRef] [PubMed]
- de Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.; Zanettini, M.H.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (HIPP): characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Tehseen, M.; Cairns, N.; Sherson, S.; Cobbett, C.S. Metallochaperone-like genes in Arabidopsis thaliana. Metallomics 2010, 2, 556–564. [Google Scholar] [CrossRef]
- Cowan, G.H.; Roberts, A.G.; Jones, S.; Kumar, P.; Kalyandurg, P.B.; Gil, J.F.; Savenkov, E.I.; Hemsley, P.A.; Torrance, L. Potato Mop-Top Virus Co-Opts the Stress Sensor HIPP26 for Long-Distance Movement. Plant Physiol. 2018, 176, 2052–2070. [Google Scholar] [CrossRef] [Green Version]
- Urano, D.; Miura, K.; Wu, Q.; Iwasaki, Y.; Jackson, D.; Jones, A.M. Plant Morphology of Heterotrimeric G Protein Mutants. Plant Cell Physiol. 2016, 57, 437–445. [Google Scholar] [CrossRef]
- Peng, P.; Gao, Y.; Li, Z.; Yu, Y.; Qin, H.; Guo, Y.; Huang, R.; Wang, J. Proteomic Analysis of a Rice Mutant sd58 Possessing a Novel d1 Allele of Heterotrimeric G Protein Alpha Subunit (RGA1) in Salt Stress with a Focus on ROS Scavenging. Int. J. Mol. Sci. 2019, 20, 167. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Feng, Y.; Long, D.; Cao, B.; Xiang, Z.; Zhao, A. Ectopic Expression of Mulberry G-Proteins Alters Drought and Salt Stress Tolerance in Tobacco. Int. J. Mol. Sci. 2018, 20, 89. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Toledo-Ortiz, G.; Yalovsky, S.; Caldelari, D.; Gruissem, W. Carboxyl-methylation of prenylated calmodulin CaM53 is required for efficient plasma membrane targeting of the protein. Plant J. 2000, 24, 775–784. [Google Scholar] [CrossRef]
- Sorek, N.; Gutman, O.; Bar, E.; Abu-Abied, M.; Feng, X.; Running, M.P.; Lewinsohn, E.; Ori, N.; Sadot, E.; Henis, Y.I.; et al. Differential effects of prenylation and s-acylation on type I and II ROPS membrane interaction and function. Plant Physiol. 2011, 155, 706–720. [Google Scholar] [CrossRef]
- Li, Z.; Kang, J.; Sui, N.; Liu, D. ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 169–179. [Google Scholar] [CrossRef]
- Ueda, T.; Yamaguchi, M.; Uchimiya, H.; Nakano, A. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 2001, 20, 4730–4741. [Google Scholar] [CrossRef]
- Ebine, K.; Fujimoto, M.; Okatani, Y.; Nishiyama, T.; Goh, T.; Ito, E.; Dainobu, T.; Nishitani, A.; Uemura, T.; Sato, M.H.; et al. A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 2011, 13, 853–859. [Google Scholar] [CrossRef]
- Inada, N.; Betsuyaku, S.; Shimada, T.L.; Ebine, K.; Ito, E.; Kutsuna, N.; Hasezawa, S.; Takano, Y.; Fukuda, H.; Nakano, A.; et al. Modulation of Plant RAB GTPase-Mediated Membrane Trafficking Pathway at the Interface Between Plants and Obligate Biotrophic Pathogens. Plant Cell Physiol. 2016, 57, 1854–1864. [Google Scholar] [CrossRef] [Green Version]
- Asaoka, R.; Uemura, T.; Ito, J.; Fujimoto, M.; Ito, E.; Ueda, T.; Nakano, A. Arabidopsis RABA1 GTPases are involved in transport between the trans-Golgi network and the plasma membrane, and are required for salinity stress tolerance. Plant J. 2013, 73, 240–249. [Google Scholar] [CrossRef]
- Sivars, U.; Aivazian, D.; Pfeffer, SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature 2003, 425, 856–859. [Google Scholar] [CrossRef]
- Lee, M.H.; Yoo, Y.J.; Kim, D.H.; Hanh, N.H.; Kwon, Y.; Hwang, I. The Prenylated Rab GTPase Receptor PRA1.F4 Contributes to Protein Exit from the Golgi Apparatus. Plant Physiol. 2017, 174, 1576–1594. [Google Scholar] [CrossRef] [Green Version]
- Opalski, K.S.; Schultheiss, H.; Kogel, K.H.; Hückelhoven, R. The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp.hordei. Plant J. 2005, 41, 291–303. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, J.; Kuzma, M.; Chalifoux, M.; Sample, A.; McArthur, C.; Uchacz, T.; Sarvas, C.; Wan, J.; Dennis, D.T.; et al. Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J. 2005, 43, 413–424. [Google Scholar] [CrossRef]
- Wang, Y.; Beaith, M.; Chalifoux, M.; Ying, J.; Uchacz, T.; Sarvas, C.; Griffiths, R.; Kuzma, M.; Wan, J.; Huang, Y. Shoot-specific down-regulation of protein farnesyltransferase (alpha-subunit) for yield protection against drought in canola. Mol. Plant 2009, 2, 191–200. [Google Scholar] [CrossRef]
- Loraine, A.E.; Yalovsky, S.; Fabry, S.; Gruissem, W. Tomato Rab1A homologs as molecular tools for studying Rab geranylgeranyl transferase in plant cells. Plant Physiol. 1996, 110, 1337–1347. [Google Scholar] [CrossRef]
- Lu, C.; Zainal, Z.; Tucker, G.A.; Lycett, G.W. Developmental abnormalities and reduced fruit softening in tomato plants expressing an antisense Rab11 GTPase gene. Plant Cell 2000, 13, 1819–1833. [Google Scholar] [CrossRef]

| Protein Name | Plant | Protein Accession | Stress | Reference |
|---|---|---|---|---|
| ANJ1 | Atriplex nummularia | P43644 | Abiotic – high temperature | [36] |
| HSP70 J2 | Arabidopsis thaliana | NP_568412 | Abiotic – high temperature, drought | [25] |
| HSP70 J3 | Arabidopsis thaliana | Q94AW8 | Abiotic – high temperature, drought | [25] |
| AtFP3 | Arabidopsis thaliana | AAD09507 | Abiotic – heavy metals | [37] |
| AtFP6 | Arabidopsis thaliana | NP_195570 | Abiotic – heavy metals | [38] |
| Cdl19 | Arabidopsis thaliana | AAM64219 | Abiotic – heavy metals | [39] |
| HvFP1 | Hordeum vulgare | Q8GTD3 | Abiotic – cold, strong light | [40] |
| AtHIPP26 | Arabidopsis thaliana | OAP00180 | Abiotic – cold, salinity | [41] |
| HIPP3 | Arabidopsis thaliana | AIE40061 | Biotic | [42] |
| RGG2 | Oryza sativa | NP_001045833 | Abiotic – drought | [43] |
| RGA1 | Oryza sativa | ABF98475 | Abiotic – salinity | [44] |
| CaM53 | Petunia x hybrida | AAA33705 | Abiotic | [45] |
| ASG2 | Arabidopsis thaliana | OAO92260 | Abiotic - salinity | [46] |
| MaROP5g | Musa acuminata | Ma09_p21130 | Abiotic - salinity | [47] |
| ROP2 | Arabidopsis thaliana | OAP19779 | Abiotic - salinity | [48] |
| ROP10 | Arabidopsis thaliana | OAP04715 | Abiotic - drought | [49] |
| RACB | Hordeum vulgare | CAC83043 | Abiotic - drought | [50] |
| RAB-G3e | Arabidopsis thaliana | NP_001031161 | Abiotic – osmotic, Biotic | [51] |
| OsRAB7 | Oryza sativa | AAO67728 | Abiotic - salinity | [52] |
| ROP6 | Arabidopsis thaliana | OAP00270 | Biotic | [53] |
| AIG1 | Arabidopsis thaliana | P54120 | Biotic | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hála, M.; Žárský, V. Protein Prenylation in Plant Stress Responses. Molecules 2019, 24, 3906. https://doi.org/10.3390/molecules24213906
Hála M, Žárský V. Protein Prenylation in Plant Stress Responses. Molecules. 2019; 24(21):3906. https://doi.org/10.3390/molecules24213906
Chicago/Turabian StyleHála, Michal, and Viktor Žárský. 2019. "Protein Prenylation in Plant Stress Responses" Molecules 24, no. 21: 3906. https://doi.org/10.3390/molecules24213906






