Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Substrates, Enzyme, and Equipment
3.2. SDS-Page Gel Analysis
3.3. Relative Hydrolysis
3.4. Sugar Analysis
3.5. Pre-Milling of Biomass
3.6. Determination of Optimal Water Loading
3.7. TLC Analysis of the Soluble Enzymatic Reaction Products
3.8. Reactions in Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirk, R.E.; Othmer, D. Encyclopedia of Chemical Technology, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Morgan, H.M., Jr.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Prade, R.A. Xylanases: From biology to biotechnology. Biotechnol. Genet. Eng. Rev. 1995, 13, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Hilpmann, G.; Becher, N.; Pahner, F.A.; Kusema, B.; Mäki-Arvela, P.; Lange, R.; Murzin, D.Y.; Salmi, T. Acid hydrolysis of xylan. Catal. Today 2016, 259, 376–380. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Luo, C.; Brink, D.L.; Blanch, H.W. Identification of potential fermentation inhibitors in conversion of hybrid poplar hydrolysate to ethanol. Biomass Bioenergy 2002, 22, 125–138. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.J.; Schwinghamer, T.; Orsat, V.; Del Rio, L.F. Model study to assess softwood hemicellulose hydrolysates as the carbon source for PHB production in Paraburkholderia sacchari IPT 101. Biomacromolecules 2018, 19, 188–200. [Google Scholar] [CrossRef]
- Kucera, D.; Benesova, P.; Ladicky, P.; Pekar, M.; Sedlacek, P.; Obruca, S. Production of polyhydroxyalkanoates using hydrolysates of spruce sawdust: Comparison of hydrolysates detoxification by application of overliming, active carbon, and lignite. Bioengineering 2017, 4, 53. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; Suzane dos Santos Milessi, T.; Antunes, F.A.F.; Luiz da Costa Freitas, W.; das Graças Almeida Felipe, M.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotecnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Perrotta, J.A.; Stipanovic, A.J.; Nomura, C.T.; Nakas, J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J. Ind. Microbiol. Biotechnol. 2012, 39, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Polizeli, M.L.T.M.; Rizzatti, A.C.S.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Zhang, J.; Siika-aho, M.; Puranen, T.; Tang, M.; Tenkanen, M.; Viikari, L. Thermostable recombinant xylanases from Nonomuraea flexuosa and Thermoascus aurantiacus show distinct properties in the hydrolysis of xylans and pretreated wheat straw. Biotechnol. Biofuels 2011, 4, 12. [Google Scholar] [CrossRef]
- Wang, K.; Cao, R.; Wang, M.; Lin, Q.; Zhan, R.; Xu, H.; Wang, S. A novel thermostable GH10 xylanase with activities on a wide variety of cellulosic substrates from a xylanolytic Bacillus strain exhibiting significant synergy with commercial Celluclast 1.5 L in pretreated corn stover hydrolysis. Biotechnol. Biofuels 2019, 12, 48. [Google Scholar] [CrossRef]
- Patipong, T.; Lotrakul, P.; Padungros, P.; Punnapayak, H.; Bankeeree, W.; Prasongsuk, S. Enzymatic hydrolysis of tropical weed xylans using xylanase from Aureobasidium melanogenum PBUAP46 for xylooligosaccharide production. 3 Biotech 2019, 9, 56. [Google Scholar] [CrossRef]
- Zhuo, R.; Yu, H.; Qin, X.; Ni, H.; Jiang, Z.; Ma, F.; Zhang, X. Heterologous expression and characterization of a xylanase and xylosidase from white rot fungi and their application in synergistic hydrolysis of lignocellulose. Chemosphere 2018, 212, 24–33. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Jiang, Z.; Liu, H.; Yang, S.; Yan, Q. Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chem. 2018, 264, 310–318. [Google Scholar] [CrossRef]
- Hernández, J.G.; Frings, M.; Bolm, C. Mechanochemical enzymatic kinetic resolution of secondary alcohols under ball-milling conditions. ChemCatChem. 2016, 8, 1769–1772. [Google Scholar] [CrossRef]
- Hernández, J.G.; Ardila-Fierro, K.J.; Crawford, D.; James, S.L.; Bolm, C. Mechanoenzymatic peptide and amide bond formation. Green Chem. 2017, 19, 2620–2625. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Crawford, D.E.; Körner, A.; James, S.L.; Bolm, C.; Hernández, J.G. Papain-catalysed mechanochemical synthesis of oligopeptides by milling and twin-screw extrusion: Application in the Juliá–Colonna enantioselective epoxidation. Green Chem. 2018, 20, 1262–1269. [Google Scholar] [CrossRef]
- Hernández, J.G.; Turberg, M.; Schiffers, I.; Bolm, C. Mechanochemical Strecker reaction: Access to α-aminonitriles and tetrahydroisoquinolines under ball-milling conditions. Chem. Eur. J. 2016, 22, 14513–14517. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Venegas, M.; Reyes-Rangel, G.; Neri, A.; Escalante, J.; Juaristi, E. Mechanochemical enzymatic resolution of N-benzylated-β3-amino esters. Beilstein J. Org. Chem. 2017, 13, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Venegas, M.; Juaristi, E. Mechanoenzymatic resolution of racemic chiral amines, a green technique for the synthesis of pharmaceutical building blocks. Tetrahedron 2018, 74, 6453–6458. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. From synthesis of amino acids and peptides to enzymatic catalysis: A bottom-up approach in mechanochemistry. ChemSusChem. 2018, 11, 1410–1420. [Google Scholar] [CrossRef]
- Hammerer, F.; Loots, L.; Do, J.L.; Therien, J.D.; Nickels, C.W.; Friščić, T.; Auclair, K. Solvent-free enzyme activity: Quick, high-yielding mechanoenzymatic hydrolysis of cellulose into glucose. Angew. Chem. 2018, 130, 2651–2654. [Google Scholar] [CrossRef]
- Hammerer, F.; Ostadjoo, S.; Friščić, T.; Auclair, K. Controlling the reactivity of enzymes in mechanochemistry: Inert surfaces protect β-glucosidase activity during ball milling. ChemSusChem 2019. [Google Scholar] [CrossRef]
- Therien, J.P.D.; Hammerer, F.; Friščić, T.; Auclair, K. Mechanoenzymatic breakdown of chitinous material to N-acetylglucosamine: The benefits of a solvent-less environment. ChemSusChem 2019, 12, 3481–3490. [Google Scholar]
- Kaabel, S.; Friščić, T.; Auclair, K. Mechanoenzymatic transformations in the absence of bulk water – A more natural way of using enzymes. ChemBioChem 2019. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Centr. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colacino, E.; Carta, M.; Pia, G.; Porcheddu, A.; Ricci, P.C.; Delogu, F. Processing and investigation methods in mechanochemical kinetics. ACS Omega 2018, 3, 9196–9209. [Google Scholar] [CrossRef] [Green Version]
- Cliffe, M.J.; Mottillo, C.; Stein, R.S.; Bučar, D.K.; Friščić, T. Accelerated aging: A low energy, solvent-free alternative to solvothermal and mechanochemical synthesis of metal–organic materials. Chem. Sci. 2012, 3, 2495–2500. [Google Scholar] [CrossRef]
- Mottillo, C.; Friščić, T. Advances in solid-state transformations of coordination bonds: From the ball mill to the aging chamber. Molecules 2017, 22, 144. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, R.G.; Shafizadeh, F. Enhancement of cellulose accessibility and enzymatic hydrolysis by simultaneous wet milling. Biotechnol. Bioeng. 1980, 22, 1025–1036. [Google Scholar] [CrossRef]
- Mais, U.; Esteghlalian, A.R.; Saddler, J.N.; Mansfield, S.D. Enhancing the enzymatic hydrolysis of cellulosic materials using simultaneous ball milling. App. Biochem. Biotechnol. 2002, 98–100, 815–832. [Google Scholar] [CrossRef]
- Hasa, D.; Miniussi, E.; Jones, W. Mechanochemical synthesis of multicomponent crystals: One liquid for one polymorph? A myth to dispel. Cryst. Growth Des. 2016, 16, 4582–4588. [Google Scholar] [CrossRef] [Green Version]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm. 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Hasa, D.; Jones, W. Screening for new pharmaceutical solid forms using mechanochemistry: A practical guide. Adv. Drug Deliv. Rev. 2017, 117, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Madlala, A.M.; Prior, B.A. Thermomyces lanuginosus: Properties of strains and their hemicellulases. FEMS Microbiol. Rev. 2003, 27, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Romero-Fernández, M.; Moreno-Perez, S.; Orrego, A.H.; de Oliveira, S.M.; Santamaría, R.I.; Díaz, M.; Guisan, J.M.; Rocha-Martin, J. Designing continuous flow reaction of xylan hydrolysis for xylooligosaccharides production in packed-bed reactors using xylanase immobilized on methacrylic polymer-based supports. Bioresour. Technol. 2018, 266, 249–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Yu, X.; Kong, H.; Chen, J.; Luo, H.; Bai, Y.; Yao, B. Synergistic effect of acetyl xylan esterase from Talaromyces leycettanus JCM12802 and xylanase from Neocallimastix patriciarum achieved by introducing carbohydrate-binding module-1. AMB Express 2019, 9, 13. [Google Scholar] [CrossRef]
- Wi, S.G.; Cho, E.J.; Lee, D.S.; Lee, S.J.; Lee, Y.J.; Bae, H.J. Lignocellulose conversion for biofuel: A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels 2015, 8, 228. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |
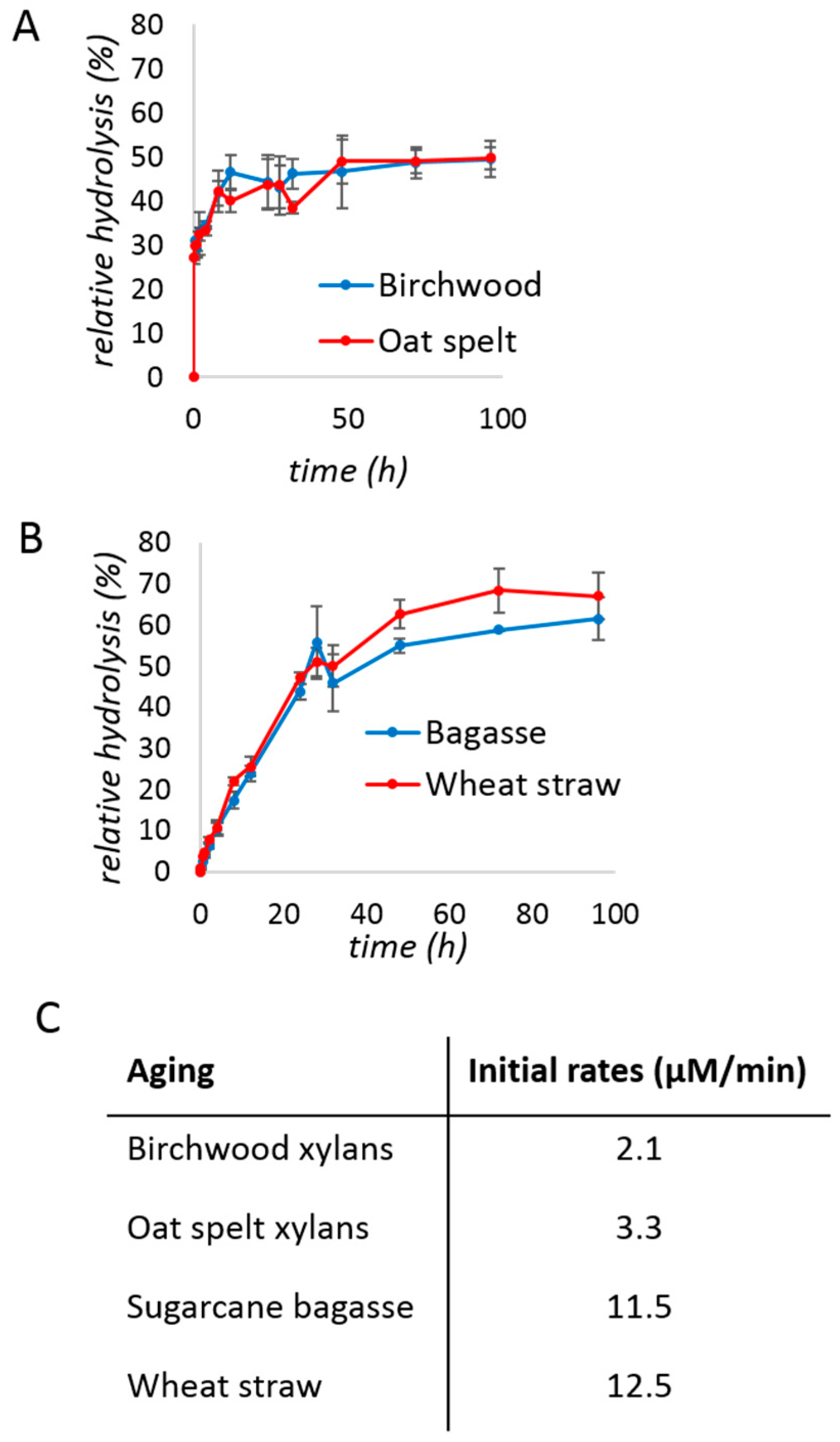
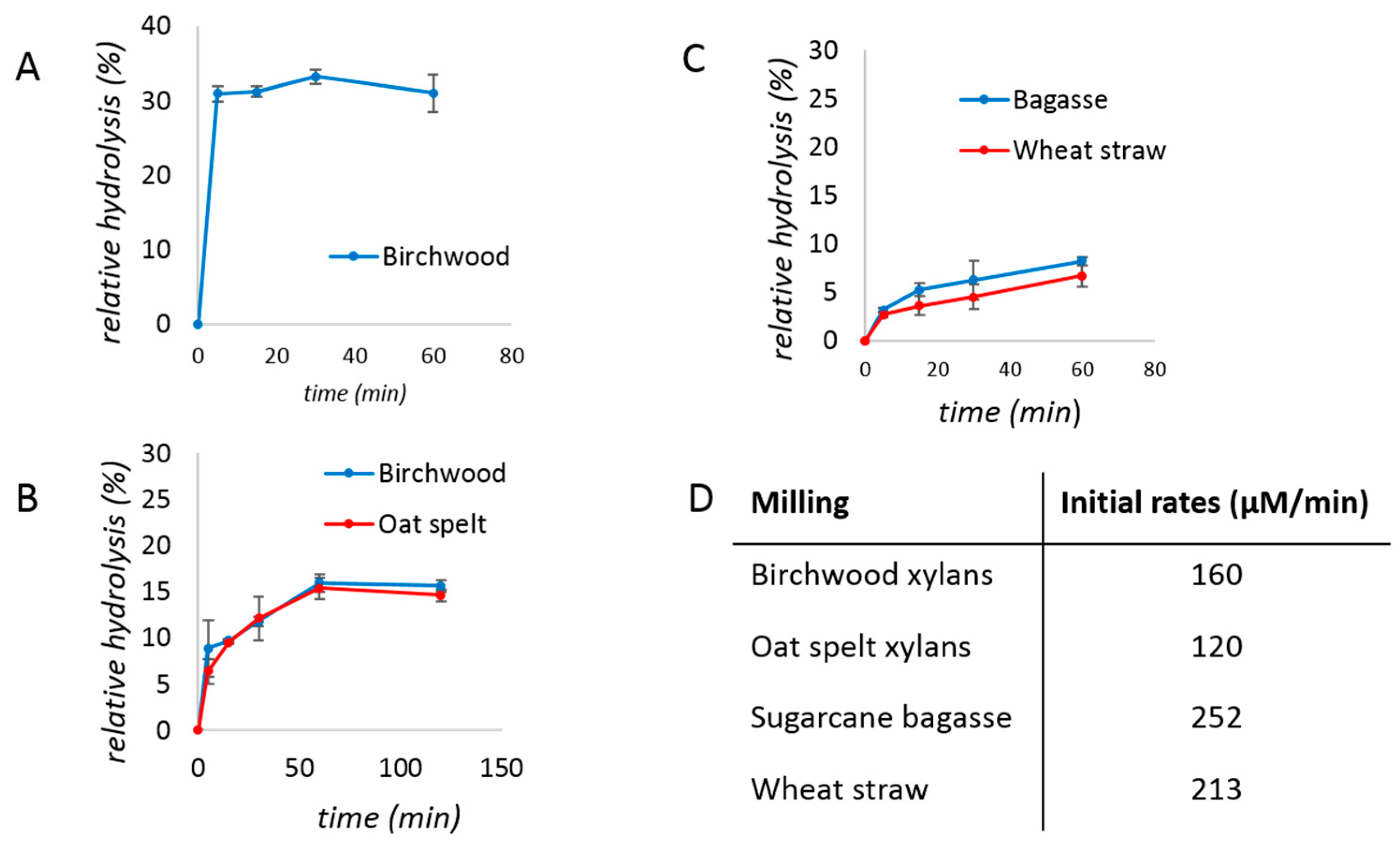
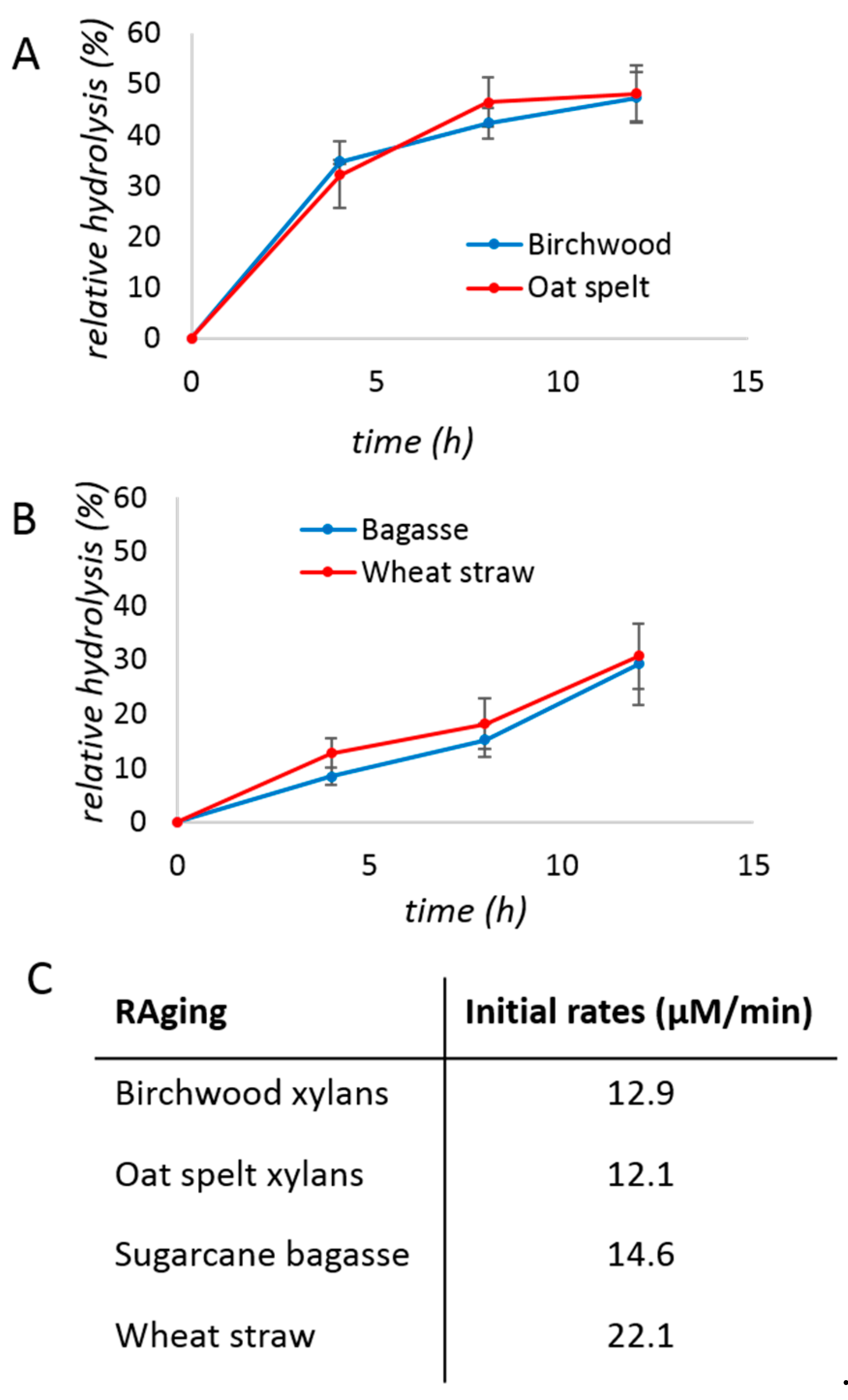
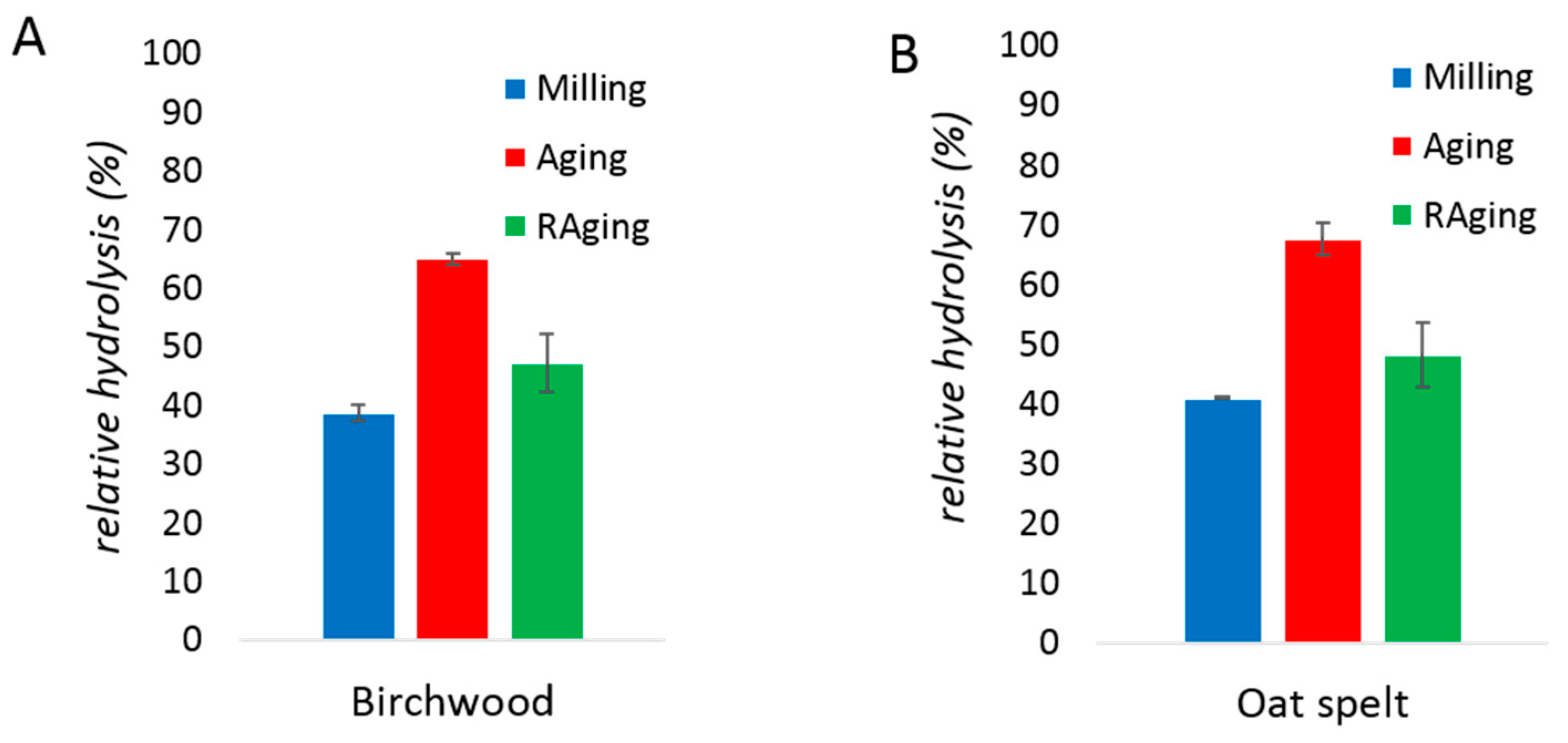


| No Bulk Water | With Bulk Water | |||
|---|---|---|---|---|
| Milling 30 min, RT | Aging 72 h, 55 °C | Shaking 30 min, RT | Shaking 72 h, 55 °C | |
| Birchwood xylan | 39 ± 1% | 65 ± 1% | 14 ± 1% | 24 ± 3% |
| Oat spelt xylan | 41 ± 1% | 68 ± 3% | 14 ± 1% | 26 ± 1% |
| Sugarcane bagasse | 12 ± 6% | 73 ± 14% | 1.6 ± 0.5% | 4 ± 1% |
| Wheat straw | 8 ± 4% | 84 ± 23% | 2.4 ± 0.2% | 5 ± 2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostadjoo, S.; Hammerer, F.; Dietrich, K.; Dumont, M.-J.; Friscic, T.; Auclair, K. Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water. Molecules 2019, 24, 4206. https://doi.org/10.3390/molecules24234206
Ostadjoo S, Hammerer F, Dietrich K, Dumont M-J, Friscic T, Auclair K. Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water. Molecules. 2019; 24(23):4206. https://doi.org/10.3390/molecules24234206
Chicago/Turabian StyleOstadjoo, Shaghayegh, Fabien Hammerer, Karolin Dietrich, Marie-Josée Dumont, Tomislav Friscic, and Karine Auclair. 2019. "Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water" Molecules 24, no. 23: 4206. https://doi.org/10.3390/molecules24234206






