A Recyclable, Metal-Free Mechanochemical Approach for the Oxidation of Alcohols to Carboxylic Acids
Abstract
:1. Introduction
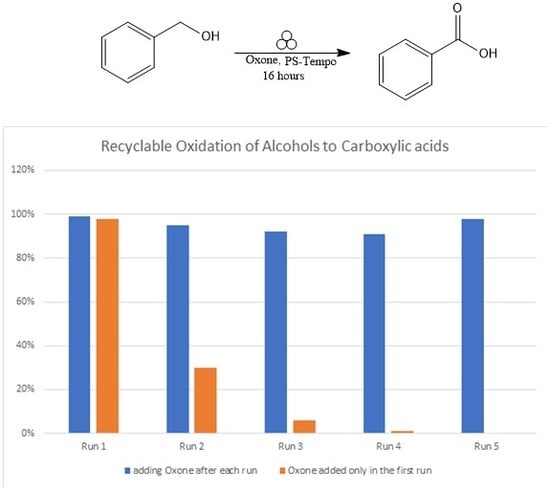
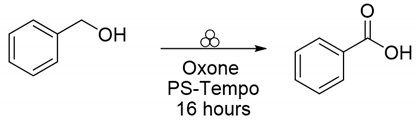
2. Results and Discussion
3. Materials and Methods
3.1. PS-TEMPO/Oxone®/Stainless Steel
3.2. Substrate Size Study
3.3. TEMPO/Copper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Note
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Adams, C.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B.; Leonhardt, S. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef]
- Do, J.L.; Friscic, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci 2017, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Tan, D.; Garcia, F. Main group mechanochemistry: From curiosity to established protocols. Chem Soc. Rev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Chacon-Huete, F.; Messina, C.; Chen, F.; Cuccia, L.; Ottenwaelder, X.; Forgione, P. Solvent-free mechanochemical oxidation and reduction of biomass-derived 5-hydroxymethyl furfural. Green Chem. 2018, 20, 5261–5265. [Google Scholar] [CrossRef]
- Bolm, C.; Hernandez, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem., Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Takahashi, R.; Ito, H. Mechanochemistry allows carrying out sensitive organometallic reactions in air: Glove-box-and-Schlenk-line-free synthesis of oxidative addition complexes from aryl halides and palladium(0). Chem. Sci. 2019, 10, 5837–5842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcheddu, A.; Delogu, F.; De Luca, L.; Fattuoni, C.; Colacino, E. Metal-free mechanochemical oxidations in Ertalyte jars. Beilstein J. Org. Chem. 2019, 15, 1786–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saberi, F.; Rodriguez-Padron, D.; Doustkhah, E.; Ostovar, S.; Franco, A.; Shaterian, H.R.; Luque, R. Mechanochemically modified aluminosilicates for efficient oxidation of vanillyl alcohol. Catal. Commun. 2019, 118, 65–69. [Google Scholar] [CrossRef]
- Sharma, B.M.; Atapalkar, R.S.; Kulkarni, A.A. Continuous flow solvent free organic synthesis involving solids (reactants/products) using a screw reactor. Green Chem. 2019, 21, 5639–5646. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Sun, Z.; Qian, P.; Han, P.; Yu, B.; Ye, S. A Novel, Solvent-Free mechanochemistry approach for gold extraction from anode slime. ACS Sustain. Chem. Eng. 2019, 7, 11415–11425. [Google Scholar] [CrossRef]
- Yu, J.; Shou, H.; Yu, W.; Chen, H.; Su, W. Mechanochemical oxidative heck coupling of activated and unactivated alkenes: A chemo-, regio- and stereo-controlled synthesis of alkenylbenzenes. Adv. Synth. Catal. 2019, 361, 5133–5139. [Google Scholar] [CrossRef]
- Mishra, A.K.; Moorthy, J.N. Mechanochemical catalytic oxidations in the solid state with in situ-generated modified IBX from 3,5-di-tert-butyl-2-iodobenzoic acid (DTB-IA)/Oxone. Org. Chem. Front. 2017, 4, 343–349. [Google Scholar] [CrossRef]
- Achar, T.K.; Maiti, S.; Mal, P. IBX works efficiently under solvent free conditions in ball milling. RSC Adv. 2014, 4, 12834–12839. [Google Scholar] [CrossRef]
- Porcheddu, A.; Colacino, E.; Cravotto, G.; Delogu, F.; de Luca, L. Mechanically induced oxidation of alcohols to aldehydes and ketones in ambient air: Revisiting TEMPO-assisted oxidations. Beilstein J. Org. Chem. 2017, 13, 2049–2055. [Google Scholar] [CrossRef] [Green Version]
- Hodge, P. Polymer-Supported organic reactions: What takes place in the beads? Chem. Soc. Rev. 1997, 26, 417–424. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Fields, G.B.; Fields, C.g. Solvation effects in solid-phase peptide synthesis. J. Am. Chem. Soc. 1991, 113, 4202–4207. [Google Scholar] [CrossRef]
- McNamara, C.A.; Dixon, M.J.; Bradley, M. Recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem. Rev. 2002, 102, 3275–3300. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.J.; Friscic, T.; Moores, A. One-step, solvent-free mechanosynthesis of silver nanoparticle-infused lignin composites for use as highly active multidrug resistant antibacterial filters. RSC Adv. 2016, 6, 58365–58370. [Google Scholar] [CrossRef] [Green Version]
- Levy, A.; Wang, F.; Lang, A.; Galant, O.; Diesendruck, C.E. Intramolecular cross-linking: Addressing mechanochemistry with a bioinspired approach. Angew. Chem. Int. Ed. 2017, 56, 6431–6434. [Google Scholar] [CrossRef]
- Shearouse, W.C.; Mack, J. Diastereoselective liquid assisted grinding: ‘cracking’ functional resins to advance chromatography-free synthesis. Green Chem. 2012, 14. [Google Scholar] [CrossRef]
- Chen, L.; Regan, M.; Mack, J. The choice is yours: Using liquid-assisted grinding to choose between products in the palladium-catalyzed dimerization of terminal alkynes. ACS Catal. 2016, 6, 868–872. [Google Scholar] [CrossRef]
- Easter, Q.T.; Trauschke, V.; Blum, S.A. Catalyst inefficiencies: Supported ring-opening metathesis polymerization catalyst yields its ensemble rate from a small number of molecular active sites. ACS Catal. 2015, 5, 2290–2295. [Google Scholar] [CrossRef]
- Murahashi, S.; Komiya, N. Modern Oxidation Methods; Backvall, J.-E., Ed.; Wiley-VCH: Weinhein, Germany, 2010. [Google Scholar]
- Tojo, G.; Fernandez, M.I. Oxidation of Alcohols to Aldehydes and Ketones; Tojo, G., Fernández, M.I., Eds.; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Tojo, G.; Fernandez, M.I. Oxidation of Primary Alcohols to Carboxylic Acids; Tojo, G., Fernández, M.I., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Tohma, H.; Takizawa, S.; Maegawa, T.; Kita, Y. Facile and clean oxidation of alcohols in water using hypervalent iodine(III) reagents. Angew. Chem. Int. Ed. Engl. 2000, 39, 1306–1308. [Google Scholar] [CrossRef]
- Tohma, H.; Maegawa, T.; Takizawa, S.; Kita, Y. Facile and clean oxidation of alcohols in water using hypervalent iodine(III) reagents. Adv. Synth. Catal. 2002, 344, 328–337. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.-M.; Fan, K.; Dai, W.-L. One-pot solvent-free synthesis of sodium benzoate from the oxidation of benzyl alcohol over novel efficient AuAg/TiO2 catalysts. Green Chem. 2011, 13, 1644–1647. [Google Scholar] [CrossRef]
- Azizi, K.; Karimi, M.; Nikbakht, F.; Heydari, A. Direct oxidative amidation of benzyl alcohols using EDTA@Cu(II) functionalized superparamagnetic nanoparticles. Appl. Catal. A Gen. 2014, 482, 336–343. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Bolm, C.; Magnus, A.S.; Hildebrand, J.P. Catalytic synthesis of aldehydes and ketones under mild conditions using Tempo/Oxone. Org. Lett. 2000, 2, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Shearouse, W.C.; Korte, C.M.; Mack, J. A two-step ball milling method synthesizes and purifies α,β-unsaturated esters. Green Chem. 2011, 13, 598–601. [Google Scholar] [CrossRef]
- Weik, S.; Nicholson, G.; Jung, G.; Rademann, J. Oxoammonium resins as metal-free, highly reactive, versatile polymeric oxidation reagents. Angew. Chem. Int. Ed. Engl. 2001, 40, 1436–1439. [Google Scholar] [CrossRef]
- Bolm, C.; Fey, T. TEMPO oxidations with a silica-supported catalyst. Chem. Commun. 1999, 1795–1796. [Google Scholar] [CrossRef]
- Dijksman, A.; Arends, I.W.C.E.; Sheldon, R.A. Polymer immobilised TEMPO (PIPO): An efficient catalyst for the chlorinated hydrocarbon solvent-free and bromide-free oxidation of alcohols with hypochlorite. Chem. Commun. 2000, 4, 271–272. [Google Scholar] [CrossRef]
- McKissic, K.S.; Caruso, J.T.; Blair, R.G.; Mack, J. Comparison of shaking versus baking: Further understanding the energetics of a mechanochemical reaction. Green Chem. 2014, 16, 1628–1632. [Google Scholar] [CrossRef]
- Bailey, W.F.; Bobbitt, J.M.; Wiberg, K.B. Mechanism of the oxidation of alcohols by oxoammonium cations. J. Org. Chem. 2007, 72, 4504–4509. [Google Scholar] [CrossRef]
- Hocking, M.B.; Bhandari, K.; Shell, B.; Smyth, T.A. Steric and pH effects on the rate of Dakin oxidation of acylphenols. J. Org. Chem. 1982, 47, 4208–4215. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kobayashi, K.; Hotta, Y. Acid-catalyzed oxidation of benzaldehydes to phenols by hydrogen peroxide. J. Org. Chem. 1984, 49, 4740–4741. [Google Scholar] [CrossRef]
- Varma, R.S.; Naicker, K.P. The Urea−Hydrogen peroxide complex: Solid-State oxidative protocols for hydroxylated aldehydes and ketones (dakin reaction), nitriles, sulfides, and nitrogen heterocycles. Org. Lett. 1999, 1, 189–192. [Google Scholar] [CrossRef]
- Bernini, R.; Coratti, A.; Provenzano, G.; Fabrizi, G.; Tofani, D. Oxidation of aromatic aldehydes and ketones by H2O2/CH3ReO3 in ionic liquids: A catalytic efficient reaction to achieve dihydric phenols. Tetrahedron 2005, 61, 1821–1825. [Google Scholar] [CrossRef]
- van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J. Org. Chem. 2006, 2, 1182–1183. [Google Scholar] [CrossRef] [Green Version]
- Leahy, K.; Mack, A.M.; Mack, J. An EcoScale comparison of mechanochemistry and solution based reactions. In Green Technologies for the Environment; Marquidia, P., Ed.; American Chemical Society: Washington, DC, USA, 2014; Volume 1186, pp. 129–137. [Google Scholar]
- Wenbao, L.; Bing, Y. A direct comparison of the mixing efficiency in solid-phase organic synthesis by single bead IR and fluorescence spectroscopy. Tetrahedron Lett. 1997, 38, 6485–6488. [Google Scholar] [CrossRef]
- Wilson, M.E.; Paech, K.; Zhou, W.-J.; Kurth, M.J. Solvent and reagent accessibility within oligo(ethylene glycol) ether [PEG] cross-linked polystyrene beads. J. Org. Chem. 1998, 63, 5094–5099. [Google Scholar] [CrossRef]
- Grøtli, M.; Rademan, J.; Groth, T.; Lubell, W.D.; Miranda, L.P.; Meldal, M. Surfactant mediated cationic and anionic suspension polymerization of PEG-based resins in silicon oil: Beaded SPOCC 1500 and POEPOP 1500. J. Comb. Chem. 2001, 3, 28–33. [Google Scholar] [CrossRef]
- Rana, S.; White, P.; Bradley, M. Influence of resin cross-linking on solid-phase chemistry. J. Comb. Chem. 2001, 3, 9–15. [Google Scholar] [CrossRef]
- Kress, J.; Zanaletti, R.; Rose, A.; Frey, J.G.; Brocklesby, W.S.; Ladlow, M.; Bradley, M. Which sites react first? Functional site distribution and kinetics on solid supports investigated using confocal raman and fluorescence microscopy. J. Comb. Chem. 2003, 5, 28–32. [Google Scholar] [CrossRef]
- Conversions in all reactions were measured by GC-MS. The conversion was calculated using the peak integrations of the retention times of the products against the starting alcohol (i.e., conversion= products (desired)/reactants and products (total). Errors in the conversion measurements were estimated by comparing the results of at least 3 integrations of each spectrum.
- Hoover, J.M.; Ryland, B.L.; Stahl, S.S. Mechanism of copper(I)/TEMPO-catalyzed aerobic alcohol oxidation. J. Am. Chem. Soc. 2013, 135, 2357–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds benzoic acid, 4-nitrobenzoic acid, 4- chlorobenzoic acid, 4-bromobenzoic acid, toluic acid, phenyl acetic acid are available from the authors. |




| Entry | Alcohol | % Yield |
|---|---|---|
| 1 | 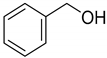 | >98 |
| 2 | 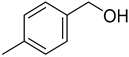 | >98 |
| 3 | 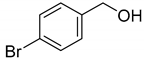 | >98 |
| 4 | 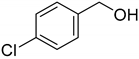 | >98 |
| 5 | 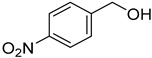 | >98 |
| 6 | 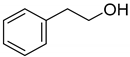 | 64 |
| 7 | 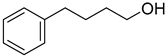 | 75 |
| 8 | 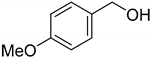 | <5 |

| Entry | Alcohol | Catalyst | % Conversion | Catalyst | % Conversion |
|---|---|---|---|---|---|
| 1 | 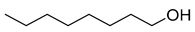 | TEMPO | 81 | PS-TEMPO | 95 |
| 2 | 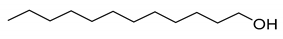 | TEMPO | 76 | PS-TEMPO | 95 |
| 3 |  | TEMPO | 80 * | PS-TEMPO | 89 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denlinger, K.L.; Carr, P.; Waddell, D.C.; Mack, J. A Recyclable, Metal-Free Mechanochemical Approach for the Oxidation of Alcohols to Carboxylic Acids. Molecules 2020, 25, 364. https://doi.org/10.3390/molecules25020364
Denlinger KL, Carr P, Waddell DC, Mack J. A Recyclable, Metal-Free Mechanochemical Approach for the Oxidation of Alcohols to Carboxylic Acids. Molecules. 2020; 25(2):364. https://doi.org/10.3390/molecules25020364
Chicago/Turabian StyleDenlinger, Kendra Leahy, Preston Carr, Daniel C. Waddell, and James Mack. 2020. "A Recyclable, Metal-Free Mechanochemical Approach for the Oxidation of Alcohols to Carboxylic Acids" Molecules 25, no. 2: 364. https://doi.org/10.3390/molecules25020364