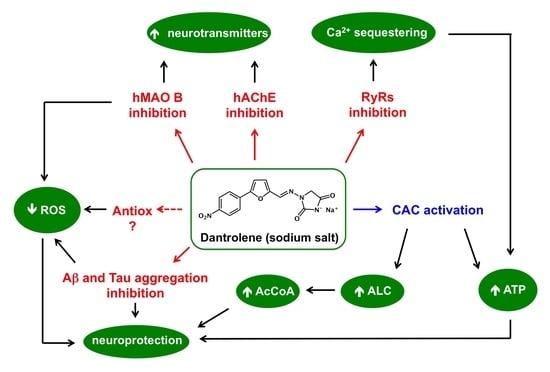
A Prospective Repurposing of Dantrolene as a Multitarget Agent for Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stability of Dantrolene in Buffered Solutions
2.2. Inhibition of MAOs and ChEs
2.3. Inhibition of Self-Aggregation of Amyloid Peptides
2.4. Radical Scavenging Activity
2.5. Cell-Based Assay of Neuroprotection
2.6. Activation of CAC Transport
3. Materials and Methods
3.1. Buffer Stability Assay
3.2. Monoamine Oxidase Inhibition Assay
3.3. Cholinesterase Inhibition Assays
3.4. Inhibition Assay of β-Amyloid Aggregation
3.5. Inhibition Assay of PHF6 Aggregation
3.6. In Vitro Radical Scavenging Assays
3.7. Cell Culture and DCF-DA Assay
3.8. CAC Activation Assay
3.8.1. Preparation of Rat Liver Mitochondria and Carnitine/Acylcarnitine Carrier (CAC) Transport Activity Measurement in Entire Organelle
3.8.2. Site-Directed Mutagenesis, Overexpression, and Isolation of the CAC Proteins
3.8.3. Reconstitution of Mitochondrial Extract or Recombinant CAC Proteins in Liposomes
3.8.4. Transport Measurements
3.8.5. EC50 Calculation and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Snyder, H.R., Jr.; Davis, C.S.; Bickerton, R.K.; Halliday, R.P. 1-[(5-arylfurfurylidene) amino]-hydantoins. A new class of muscle relaxants. J. Med. Chem. 1967, 10, 807–810. [Google Scholar] [CrossRef]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet. J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Krause, T.; Gerbershagen, M.U.; Fiege, M.; Weisshorn, R.; Wappler, F. Dantrolene—A review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004, 59, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Van Petegem, F. Ryanodine Receptors: Structure and Function. J. Biol. Chem. 2012, 287, 31624–31632. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.A.; Anderson, K.; Rousseau, E.; Liu, Q.Y.; Meissner, G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem. Biophys. Res. Commun. 1988, 151, 441–449. [Google Scholar] [CrossRef]
- Nakayama, R.; Yano, T.; Ushijima, K.; Abe, E.; Terasaki, H. Effects of dantrolene on extracellular glutamate concentration and neuronal death in the rat hippocampal CA1 region subjected to transient ischemia. Anesthesiology 2002, 96, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nguyen, H.N.; Maguire, J.L.; Perry, D.C. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J. Cereb. Blood Flow Metab. 2002, 22, 206–214. [Google Scholar] [CrossRef]
- Moon, J.; Cros, J. Role of dantrolene in the management of the acute toxic effects of Ecstasy (MDMA). Br. J. Anaesth. 2007, 99, 146. [Google Scholar] [CrossRef]
- Song, B.J.; Moon, K.H.; Upreti, V.V.; Eddington, N.D.; Lee, I.J. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr. Pharm. Biotechnol. 2010, 11, 434–443. [Google Scholar] [CrossRef]
- Alves, E.; Summavielle, T.; Alves, C.J.; Gomes da Silva, J.; Barata, J.C.; Fernandes, E.; Bastos, M.D.L.; Tavares, M.A.; Carvalho, F. Monoamine oxidase-B mediates ecstasy-induced neurotoxic effects to adolescent rat brain mitochondria. J. Neurosci. 2007, 27, 10203–10210. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Edmonson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Sheetal, A.; Manthab, A.K.; Kumar, V. Recent developments on the structure-activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683. [Google Scholar] [CrossRef]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 2013, 27, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Binde, C.D.; Tvete, I.F.; Gasemyr, J.; Natvig, B.; Klemp, M. A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson’s disease. Br. J. Clin. Pharmacol. 2018, 84, 1917–1927. [Google Scholar] [CrossRef]
- Deshwal, S.; Di Sante, M.; Di Lisa, F.; Kaludercic, N. Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr. Opin. Pharmacol. 2017, 33, 64–69. [Google Scholar] [CrossRef]
- Youdim, M.B.H. Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases. J. Neural Transm. 2018, 125, 1719–1733. [Google Scholar] [CrossRef]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in repositioning Alzheimer’s disease drugs based on a multitarget strategy. Future Med. Chem. 2016, 8, 2113–2142. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, S.H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef]
- Binda, C.; Aldeco, M.; Geldenhuys, W.J.; Tortorici, M.; Mattevi, A.; Edmondson, D.E. Molecular insights into human monoamine oxidase B inhibition by the glitazone anti-diabetes drugs. ACS Med. Chem. Lett. 2011, 3, 39–42. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target enzyme in Alzheimer’s disease: Acetylcholinesterase inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H. Reconsideration of anticholinesterase therapeutic strategies against Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Knez, D.; Sova, M.; Košak, U.; Gobec, S. Dual inhibitors of cholinesterases and monoamine oxidases for Alzheimer’s disease. Future Med. Chem. 2017, 9, 811–832. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Catto, M.; Leonetti, F.; Nicolotti, O.; Stefanachi, A.; Campagna, F.; Carotti, A. Targeting monoamine oxidases with multipotent ligands: An emerging strategy in the search of new drugs against neurodegenerative diseases. Curr. Med. Chem. 2011, 18, 4568–4587. [Google Scholar] [CrossRef] [PubMed]
- Aracena-Parks, P.; Goonasekera, S.A.; Gilman, C.P.; Dirksen, R.T.; Cecilia Hidalgo, C.; Hamilton, S.L. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J. Biol. Chem. 2006, 281, 40354–40368. [Google Scholar] [CrossRef]
- Pietrocola, F.; Galluzzi, L.; Bravo San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P.; Console, L.; Palmieri, F. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef]
- Muoio, D.M.; Noland, R.C.; Kovalik, J.P.; Seiler, S.E.; Davies, M.N.; DeBalsi, K.L.; Ilkayeva, O.R.; Stevens, R.D.; Kheterpal, I.; Zhang, J.; et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012, 15, 764–777. [Google Scholar] [CrossRef]
- Büyükokuroğlu, M.E.; GülçIn, I.; Oktay, M.; Küfrevioğlu, O.I. In vitro antioxidant properties of dantrolene sodium. Pharmacol. Res. 2001, 44, 491–494. [Google Scholar] [CrossRef]
- Pisani, L.; Iacobazzi, R.M.; Catto, M.; Rullo, M.; Farina, R.; Denora, N.; Cellamare, S.; Altomare, C.D. Investigating alkyl nitrates as nitric oxide releasing precursors of multitarget acetylcholinesterase-monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2019, 161, 292–309. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feartherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pisani, L.; Catto, M.; De Palma, A.; Farina, R.; Cellamare, S.; Altomare, C.D. Discovery of potent dual binding site acetylcholinesterase inhibitors via homo- and heterodimerization of coumarin-based moieties. ChemMedChem 2017, 12, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Chong, F.P.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Tau proteins and tauopathies in Alzheimer’s disease. Cell. Mol. Neurobiol. 2018, 38, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Cellamare, S.; Stefanachi, A.; Stolfa, D.A.; Basile, T.; Catto, M.; Campagna, F.; Sotelo, E.; Acquafredda, P.; Carotti, A. Design, synthesis, and biological evaluation of glycine-based molecular tongs as inhibitors of Abeta1-40 aggregation in vitro. Bioorg. Med. Chem. 2008, 16, 4810–4822. [Google Scholar] [CrossRef]
- Dammers, C.; Yolcu, D.; Kukuk, L.; Willbold, D.; Pickhardt, M.; Mandelkow, E.; Horn, A.H.C.; Heinrich Sticht, H.; Malhis, M.N.; Will, N.; et al. Selection and characterization of Tau binding D-enantiomeric peptides with potential for therapy of Alzheimer disease. PLoS ONE 2016, 11, e0167432. [Google Scholar] [CrossRef] [Green Version]
- Pisani, L.; De Palma, A.; Giangregorio, N.; Miniero, D.V.; Pesce, P.; Nicolotti, O.; Campagna, F.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4-isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J. Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef]
- Convertino, M.; Pellarin, R.; Catto, M.; Carotti, A.; Caflisch, A. 9,10-Anthraquinone hinders beta-aggregation: How does a small molecule interfere with Abeta-peptide amyloid fibrillation? Protein Sci. 2009, 18, 792–800. [Google Scholar]
- Cesari, I.; Hoerlé, M.; Simoes-Pires, C.; Grisoli, P.; Queiroz, E.F.; Dacarro, C.; Marcourt, L.; Moundipa, P.F.; Carrupt, P.A.; Cuendet, M.; et al. Anti-inflammatory, antimicrobial and antioxidant activities of Diospyros bipindensis (Gürke) extracts and its main constituents. J. Ethnopharmacol. 2013, 146, 264–270. [Google Scholar] [CrossRef]
- Ganguly, U.; Ganguly, A.; Sen, O.; Ganguly, G.; Cappai, R.; Argyadip Sahoo, A.; Chakrabarti, S. Dopamine cytotoxicity on SH-SY5Y cells: Involvement of α-synuclein and relevance in the neurodegeneration of sporadic Parkinson’s disease. Neurotox. Res. 2019, 35, 898–907. [Google Scholar] [CrossRef]
- Fowler, C.J.; Benedetti, M.S. The metabolism of dopamine by both forms of monoamine oxidase in the rat brain and its inhibition by cimoxatone. J. Neurochem. 1983, 40, 1534–1541. [Google Scholar] [CrossRef]
- Bonnet, U. Moclobemide: Evolution, pharmacodynamic, and pharmacokinetic properties. CNS Drug Rev. 2002, 8, 283–308. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, N.; Palmieri, F.; Indiveri, C. Glutathione controls the redox state of the mitochondrial carnitine/acylcarnitine carrier Cys residues by glutathionylation. Biochim. Biophys. Acta 2013, 1830, 5299–5304. [Google Scholar] [CrossRef] [PubMed]
- Tonazzi, A.; Giangregorio, N.; Console, L.; De Palma, A.; Indiveri, C. Nitric oxide inhibits the mitochondrial carnitine/acylcarnitine carrier through reversible S-nitrosylation of cysteine 136. Biochim. Biophys. Acta 2017, 1858, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, N.; Tonazzi, A.; Console, L.; Lorusso, I.; De Palma, A.; Indiveri, C. The mitochondrial carnitine/acylcarnitine carrier is regulated by hydrogen sulfide via interaction with C136 and C155. Biochim. Biophys. Acta 2016, 1860, 20–27. [Google Scholar] [CrossRef]
- Tonazzi, A.; Console, L.; Indiveri, C. Inhibition of mitochondrial carnitine/acylcarnitine transporter by H2O2: Molecular mechanism and possible implication in pathophysiology. Chem. Biol. Interact. 2013, 203, 423–429. [Google Scholar] [CrossRef]
- Flewellen, E.H.; Nelson, T.E.; Jones, W.P.; Arens, J.F.; Wagner, D.L. Dantrolene dose response in awake man: Implications for management of malignant hyperthermia. Anesthesiology 1983, 59, 275–280. [Google Scholar] [CrossRef]
- Zhao, F.; Li, P.; Chen, S.W.; Louis, C.F.; Fruen, B.R. Dantrolene inhibition of ryanodine receptor Ca2+ release channels: Molecular mechanism and isoform selectivity. J. Biol. Chem. 2001, 276, 13810–13816. [Google Scholar] [CrossRef] [Green Version]
- Kido, Y.; Tamai, I.; Ohnari, A.; Sai, Y.; Kagami, T.; Nezu, J.; Nikaido, H.; Hashimoto, N.; Asano, M.; Tsuji, A. Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-L-carnitine across the blood-brain barrier. J. Neurochem. 2001, 79, 959–969. [Google Scholar] [CrossRef]
- Alves, E.; Binienda, Z.; Carvalho, F.; Alves, J.; Fernandes, E.; Bastos, M.L.; Tavares, M.A.; Summavielle, T. Acetyl-l-carnitine provides effective in vivo neuroprotection. Neuroscience 2009, 158, 514–523. [Google Scholar] [CrossRef]
- Virmani, A.; Binienda, Z. Role of carnitine esters in brain neuropathology. Mol. Aspects Med. 2004, 25, 533–549. [Google Scholar] [CrossRef]
- Arduini, A.; Bonomini, M.; Savica, V.; Amato, A.; Zammit, V. Carnitine in metabolic disease: Potential for pharmacological intervention. Pharmacol. Ther. 2008, 120, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Purgatorio, R.; de Candia, M.; Catto, M.; Carrieri, A.; Pisani, L.; De Palma, A.; Toma, M.; Ivanova, O.A.; Voskressensky, L.G.; Altomare, C.D. Investigating 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Catto, M.; Arnesano, F.; Palazzo, G.; De Stradis, A.; Calò, V.; Losacco, M.; Purgatorio, R.; Campagna, F. Investigation on the influence of (Z)-3-(2-(3-chlorophenyl)hydrazono)-5,6-dihydroxyindolin-2-one (PT2) on β-amyloid(1–40) aggregation and toxicity. Arch. Biochem. Biophys. 2014, 560, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, M.R.; Giorgi, C.; Lebiedzinska, M.; Duszynski, J.; Pinton, P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 2009, 4, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Scalera, V.; Storelli, C.; Storelli-Joss, C.; Haase, W.; Murer, H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem. J. 1980, 186, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Pande, S.V. A mitochondrial carnitine acylcarnitine translocase system. Proc. Natl. Acad. Sci. USA 1975, 72, 883–887. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, F.; Indiveri, C.; Bisaccia, F.; Iacobazzi, V. Mitochondrial metabolite carrier proteins: Purification, reconstitution, and transport studies. Meth. Enzymol. 1995, 260, 349–369. [Google Scholar]
- Indiveri, C.; Iacobazzi, V.; Giangregorio, N.; Palmieri, F. Bacterial overexpression, purification, and reconstitution of the carnitine/acylcarnitine carrier from rat liver mitochondria. Biochem. Biophys. Res. Commun. 1998, 249, 589–594. [Google Scholar] [CrossRef]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- EC50 Calculator. Available online: https://www.aatbio.com/tools/ec50-calculator/ (accessed on 23 October 2019).
- Heard, D.S.; Tuttle, C.S.L.; Lautenschlager, N.T.; Maier, A.B. Repurposing proteostasis-modifying drugs to prevent or treat age-related dementia: A systematic review. Front. Physiol. 2018, 9, 1520. [Google Scholar] [CrossRef]
- Wang, D.W.; Mokhonova, E.I.; Kendall, G.C.; Becerra, D.; Naeini, Y.B.; Cantor, R.M.; Spencer, M.J.; Nelson, S.F.; Miceli, M.C. Repurposing dantrolene for long-term combination therapy to potentiate antisense-mediated DMD exon skipping in the mdx mouse. Mol. Ther. Nucleic Acids 2018, 11, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebouche, C.J. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bussiere, R.; Lacampagne, A.; Steven Reiken, S.; Liu, X.; Scheuerman, V.; Zalk, R.; Martin, C.; Checler, F.; Marks, A.R.; Chami, M. Amyloid β production is regulated by β2-adrenergic signaling-mediated post-translational modifications of the ryanodine receptor. J. Biol. Chem. 2017, 292, 10153–10168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease—A therapeutic opportunity? Biochem. Biophys. Res. Commun. 2017, 483, 998–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

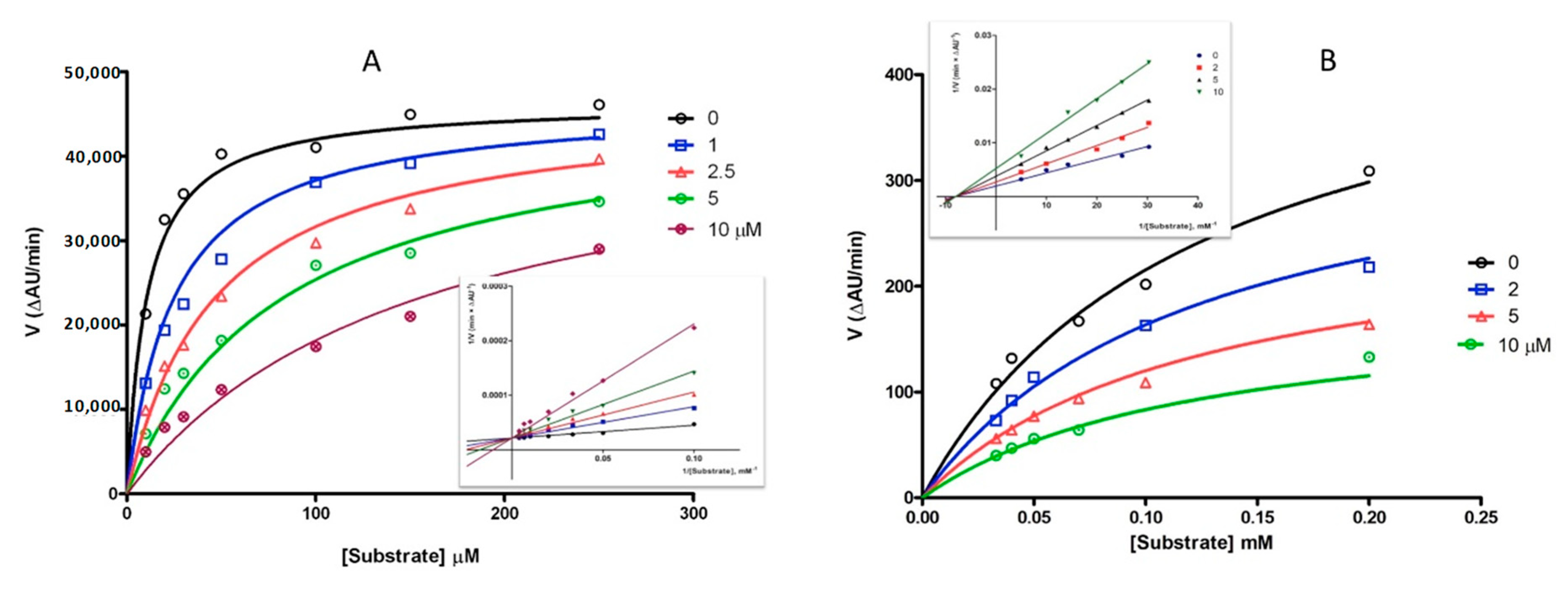






| hMAO B | hMAO A | hAChE | hBChE | |||
|---|---|---|---|---|---|---|
| IC50 | Ki | IC50 | IC50 | Ki | IC50 | |
| Dantrolene | 2.69 ± 0.44 | 0.96 ± 0.09 (comp.) | 14.0 ± 1.0 | 4.19 ± 0.73 | 6.30 ± 0.15 (noncomp.) | No inhibition |
| Pargyline | 2.69 ± 0.48 | - | 10.9 ± 0.6 | - | - | - |
| Galantamine | - | - | - | 0.62 ± 0.13 | - | 8.78 ± 0.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolognino, I.; Giangregorio, N.; Pisani, L.; de Candia, M.; Purgatorio, R.; Tonazzi, A.; Altomare, C.D.; Cellamare, S.; Catto, M. A Prospective Repurposing of Dantrolene as a Multitarget Agent for Alzheimer’s Disease. Molecules 2019, 24, 4298. https://doi.org/10.3390/molecules24234298
Bolognino I, Giangregorio N, Pisani L, de Candia M, Purgatorio R, Tonazzi A, Altomare CD, Cellamare S, Catto M. A Prospective Repurposing of Dantrolene as a Multitarget Agent for Alzheimer’s Disease. Molecules. 2019; 24(23):4298. https://doi.org/10.3390/molecules24234298
Chicago/Turabian StyleBolognino, Isabella, Nicola Giangregorio, Leonardo Pisani, Modesto de Candia, Rosa Purgatorio, Annamaria Tonazzi, Cosimo Damiano Altomare, Saverio Cellamare, and Marco Catto. 2019. "A Prospective Repurposing of Dantrolene as a Multitarget Agent for Alzheimer’s Disease" Molecules 24, no. 23: 4298. https://doi.org/10.3390/molecules24234298