Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production
Abstract
:1. Introduction
2. Results
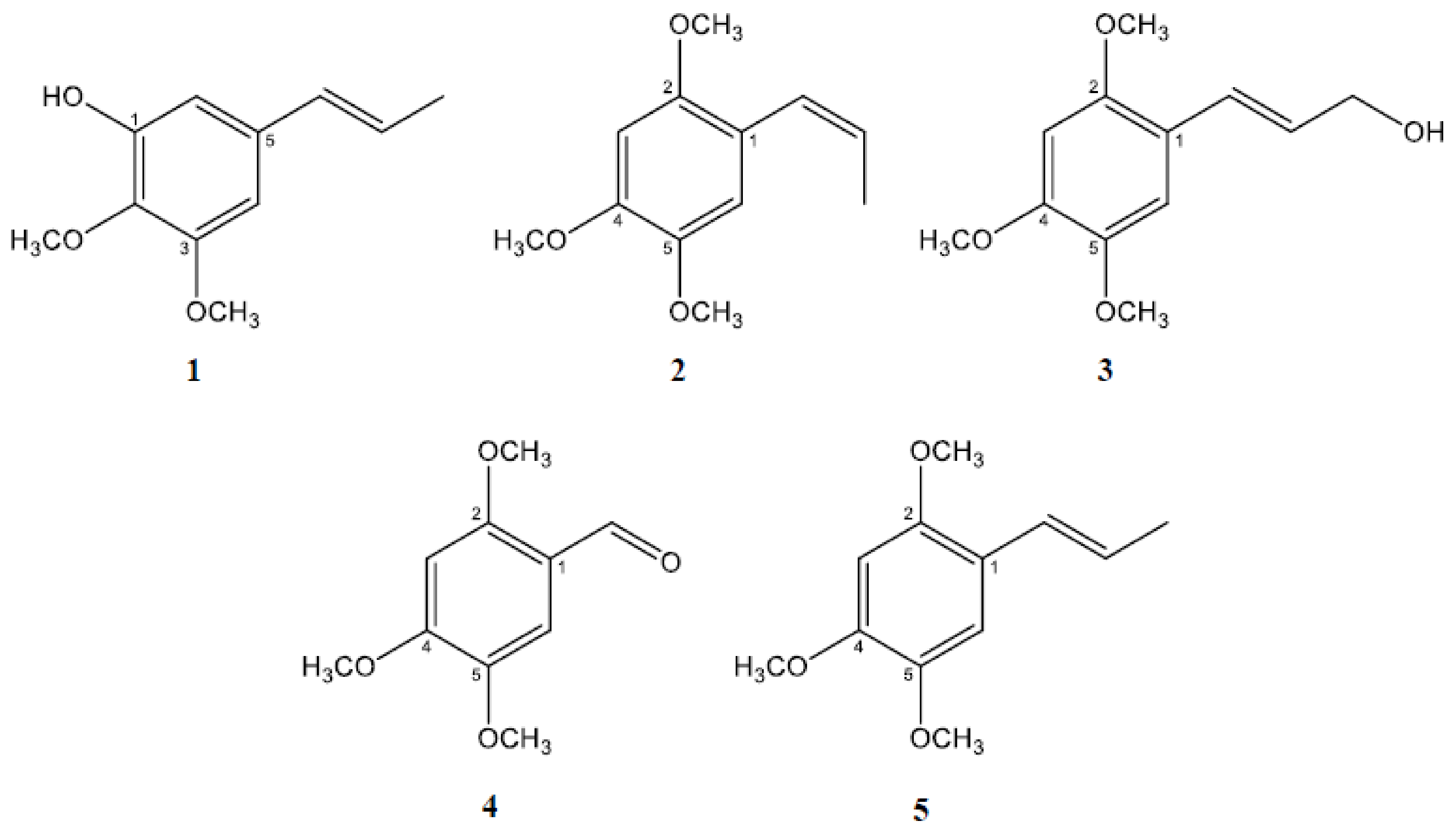
2.1. Isolation and Characterization of the Active Constituents Inhibiting Aβ Aggregation
2.2. Asarone Derivatives Inhibit Aβ Aggregation
2.3. Asarone Derivatives Increase the Disaggregation of Pre-Aggregated Aβ
2.4. Asarone Derivatives Protect PC12 Cells from Aβ-Induced Toxicity
2.5. The Inhibition of Aβ Aggregation by Asarone Derivatives Rescues PC12 Cells from Aβ Toxicity
2.6. Asarone Derivatives Reduce NO Production in LPS-Stimulated Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Extracts and Isolation
4.4. Thioflavin T (ThT) Assay
4.5. Cell Viabiltiy Assay
4.6. Determination of NO production
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992, 256, 184. [Google Scholar] [CrossRef]
- Mudher, A.; Lovestone, S. Alzheimer’s disease–do tauists and baptists finally shake hands? Trends Neurosci. 2002, 25, 22–26. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Olsson, F.; Schmidt, S.; Althoff, V.; Munter, L.M.; Jin, S.; Rosqvist, S.; Lundkvist, J. Characterization of Intermediate Steps in Amyloid Beta (Aβ) Production under Near-native Conditions. J. Bio. Chem. 2014, 289, 1540–1550. [Google Scholar] [CrossRef]
- Yang, S.G.; Wang, W.Y.; Ling, T.J.; Feng, Y.; Du, X.T.; Zhang, X.; Liu, R.T. Alpha-tocopherol quinone inhibits beta-amyloid aggregation and cytotoxicity, disaggregates preformed fibrils and decreases the production of reactive oxygen species, NO and inflammatory cytokines. Neurochem. Int. 2010, 57, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla Frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Woo, K.W.; Lee, K.R.; Lee, S.K.; Kim, H.P. Inhibition of proinflammatory cytokine generation in lung inflammation by the leaves of Perilla frutescens and its constituents. Biomol. Ther. 2014, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Furuta, Y.; Wakushima, H.; Fujii, H.; Saito, K.I.; Kano, Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytoth. Res. 2003, 17, 240–243. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol. Pharm. Bull. 2003, 26, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Hur, J.M.; Yang, E.J.; Jun, M.; Park, H.J.; Lee, K.B.; Song, K.S. β-Secretase (BACE1) inhibitors from Perilla frutescens var. acuta. Arch. Pharm. Rer. 2008, 31, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, M.S.; Kim, S.; Hwang, K.W.; Park, S.Y. Anti-amyloidogenic effects of Perilla frutescens var. acutaon beta-amyloid aggregation and disaggregation. J. Food Biochem. 2017, 41, e12393. [Google Scholar] [CrossRef]
- Sairafianpour, M.; Kayser, O.; Christensen, J.; Asfa, M.; Witt, M.; Stærk, D.; Jaroszewski, J.W. Leishmanicidal and Antiplasmodial Activity of Constituents of Smirnowia i ranica. J. Nat. Prod. 2002, 65, 1754–1758. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.Y.; Yun, B.S.; Hwang, B.K. Antifungal activity of β-asarone from rhizomes of Acorus gramineus. J. Agr. Food Chem. 2004, 52, 776–780. [Google Scholar] [CrossRef]
- Cartus, A.T.; Stegmuller, S.; Simson, N.; Wahl, A.; Neef, S.; Kelm, H.; Schrenk, D. Hepatic Metabolism of Carcinogenic β-asarone. Chem. Res. Toxicol. 2015, 28, 1760–1773. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree. J. Agr. Food Chem. 2003, 51, 3865–3873. [Google Scholar] [CrossRef]
- Siergiejczyk, L.; Poplawski, J.; Lozowicka, B.; Dubis, A.; Lachowska, B. 1H and 13C NMR spectral analysis of (E)-asarone and its isomers. Mag. Reson. Chem. 2000, 38, 1037–1038. [Google Scholar] [CrossRef]
- Kirkitadze, M.D.; Bitan, G.; Teplow, D.B. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J. Neurosci. Res. 2002, 69, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N–terminus of β–amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Sisodia, S.S.; Koo, E.H.; Beyreuther, K.; Unterbeck, A.; Price, D.L. Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science 1990, 248, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Nisha, N. Phytomedicines as potential inhibitors of β amyloid aggregation: significance to Alzheimer’s disease. Chin. J. Nat. Med. 2014, 12, 801–818. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcuminlipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N. Synthetic Curcumin Analogs as Inhibitors of β -Amyloid Peptide Aggregation: Potential Therapeutic and Diagnostic Agents for Alzheimer’s Disease. Mini. Rev. Med. Chem. 2015, 15, 1110–1121. [Google Scholar] [CrossRef]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic acid attenuates β-amyloid-induced oxidative stress via Akt/GSK-3β/Fyn-mediated Nrf2 activation in PC12 cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Carrillo-Hormaza, L.; Osorio, E.; Cardona-Gómez, G.P. Effects of biflavonoids from Garcinia madruno on a triple transgenic mouse model of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 128–138. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory activity on amyloid-β aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J. Agr. Food Chem. 2006, 53, 8762–8768. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Lin, B.; Cheng, Z.Y.; Bai, M.; Shi, S.; Huang, X.X.; Song, S.J. Phenylpropanoids and lignans from Prunus tomentosa seeds as efficient β-amyloid (Aβ) aggregationinhibitors. Bioorg. Chem. 2019, 84, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.J.; Wang, G.; Liu, J.C.; Dong, M.X.; Li, X.M.; Zhang, C.; Zhou, L.; Wang, R.; Niu, Y.C. Beta-asarone attenuates beta-amyloid-induced apoptosis through the inhibition of the activation of apoptosis signal-regulating kinase 1 in SH-SY5Y cells. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 44–51. [Google Scholar]
- Park, C.H.; Kim, K.H.; Lee, I.K.; Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, K.R. Phenolic constituents of Acorus gramineus. Arch. Pharm. Res. 2011, 34, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, G.; Salazar, M.; Tamariz, J.; Labarrios, F. Dominant lethal study of alpha-asarone in male and female mice after sub-chronic treatment. Phytother 1999. [Google Scholar] [CrossRef]
- Bhat, S.D.; Ashok, B.K.; Acharya, R.N.; Ravishankar, B. Anticonvulsant activity of raw and classically processed Vacha (Acorus calamus Linn.) rhizomes. Ayu 2012, 33, 119–122. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V.; Mohamed, Z. Biphasic Effects of α-Asarone on Immobility in the Tail Suspension Test: Evidence for the Involvement of the Noradrenergic and Serotonergic Systems in Its Antidepressant-Like Activity. Front. Pharmacol. 2016, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Sur, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Alpha-Asarone, a Major Component of Acorus gramineus, Attenuates Corticosterone-Induced Anxiety-Like Behaviours via Modulating TrkB Signaling Process. Korean J. Physiol. Pharmacol. 2014, 18, 191–200. [Google Scholar] [CrossRef] [Green Version]
- An, H.M.; Li, G.W.; Lin, C.; Gu, C.; Jin, M.; Sun, W.X.; Qiu, M.F.; Hu, B. Acorus tatarinowii Schott extract protects PC12 cells from amyloid-beta induced neurotoxicity. Die Pharm. Int. J. Pharm. Sci. 2014, 69, 391–395. [Google Scholar]
- Mdina-Franco, J.L.; Lopez-Vallejo, F.; Rodriguez-Morales, S.; Castillo, R.; Chamorro, G.; Tamariz, J. Molecular docking of the highly hypolipidemic agent alpha-asarone with catalytic portion of HMG-CoA reductase. Bioorg. Med. Chem. Lett. 2005, 15, 989–994. [Google Scholar] [CrossRef]
- Feng, X.-L.; Yu, Y.; Qin, D.-P.; Gao, H.; Yao, X.-S. Acorus Linnaeus: A review of traditional uses, phytochemistry and neuropharmacology. RSC Adv. 2015, 5, 5173–5182. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, W.C.; Li, H.; Mao, S.J. Asarone injection in treating respiratory disease: A meta-analysis. Chin. J. Evid. -Based Med. 2010, 10, 1174–1181. [Google Scholar]
- Liu, L.; Wang, J.; Shi, L.; Zhang, W.; Du, X.; Wang, Z.; Zhang, Y. beta-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine 2013, 20, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.W.; Miller, E.C.; Miller, J.A.; Liem, A. Structure-activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C58BL/6 x C3H/He J F1 mice. Cancer Res. 1987, 47, 2275–2283. [Google Scholar] [PubMed]
- Chen, Q.X.; Miao, J.K.; Li, C.; Li, X.W.; Wu, X.M.; Zhang, X.P. Anticonvulsant activitiy of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol. Pharm. Bull. 2013, 36, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Phytomedicin 2017, 32, 41–58. [Google Scholar] [CrossRef]
- Li, C.; Xing, G.; Dong, M.; Zhou, L.; Li, J.; Wang, G.; Niu, Y. Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur. J. Pharmacol. 2010, 635, 96–102. [Google Scholar] [CrossRef]
- Deng, M.; Huang, L.; Ning, B.; Wang, N.; Zhang, Q.; Zhu, C.; Fang, Y. β-asarone improves learning and memory and reduces Acetyl Cholinesterase and Beta-amyloid42 levels in APP/PS1 transgenic mice by regulating Beclin-1-dependent autophagy. Brain Res. 2016, 1652, 188–194. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Choi, D.K. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef]
- Lee, S.R.; Kim, M.S.; Kim, S.; Hwang, K.W.; Park, S.Y. Constituents from Scutellaria barbata Inhibiting Nitric OxideProduction in LPS-Stimulated Microglial Cells. Chem. Biodivers. 2017, 14, e1700231. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2 and 5 are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Kim, N.; Yeo, J.Y.; Seo, D.-G.; Kim, S.; Lee, J.-S.; Hwang, K.W.; Park, S.-Y. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules 2019, 24, 4297. https://doi.org/10.3390/molecules24234297
Lee JE, Kim N, Yeo JY, Seo D-G, Kim S, Lee J-S, Hwang KW, Park S-Y. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules. 2019; 24(23):4297. https://doi.org/10.3390/molecules24234297
Chicago/Turabian StyleLee, Jae Eun, Nayeon Kim, Ji Yun Yeo, Dae-Gun Seo, Sunggun Kim, Jae-Sun Lee, Kwang Woo Hwang, and So-Young Park. 2019. "Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves Against Beta-Amyloid Aggregation and Nitric Oxide Production" Molecules 24, no. 23: 4297. https://doi.org/10.3390/molecules24234297




