The Application of Ferric Chloride-Lignin Sulfonate as Shale Inhibitor in Water-Based Drilling Fluid
Abstract
:1. Introduction
2. Results and Discussion
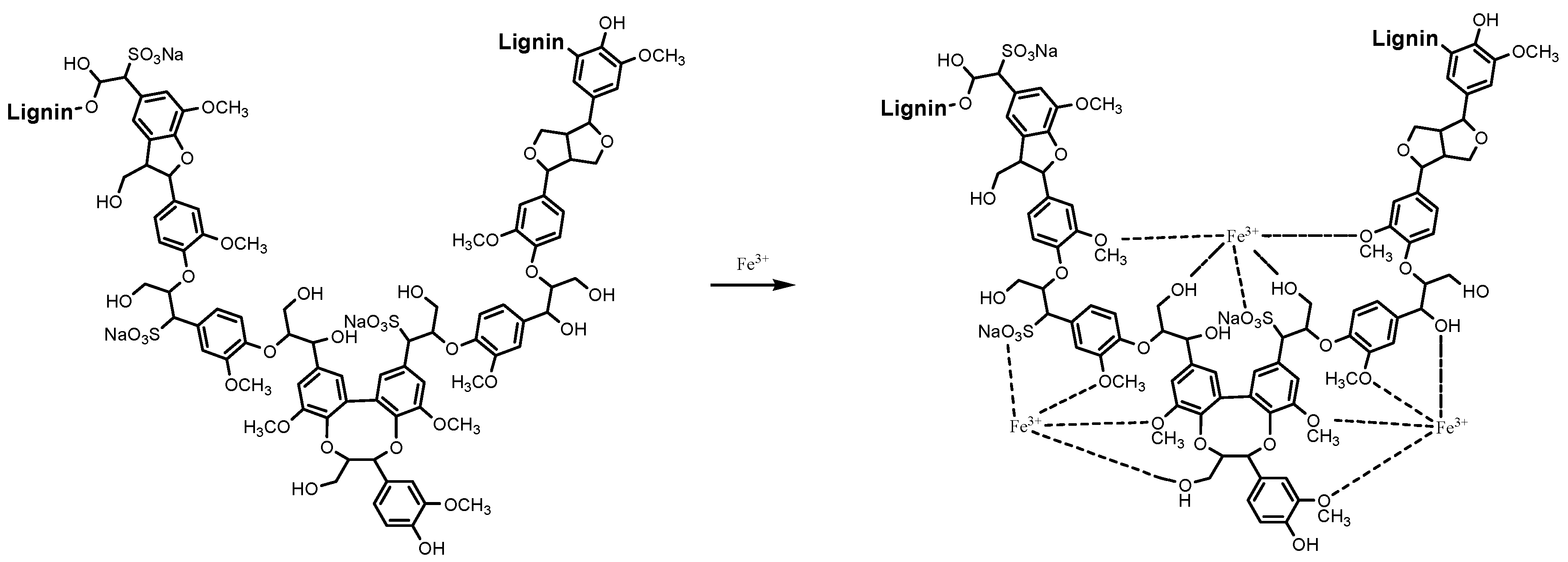
2.1. Optimization of Reaction Conditions
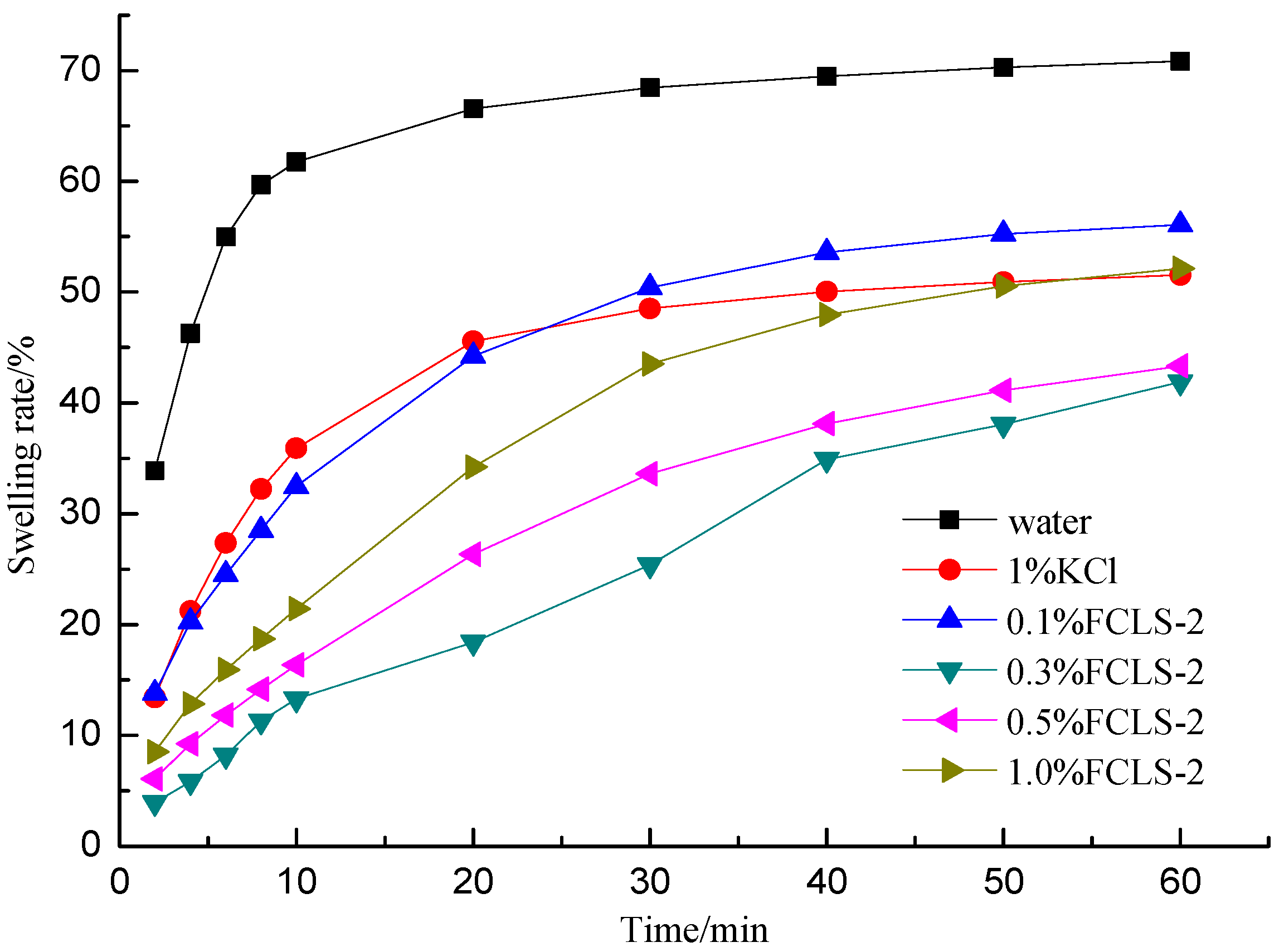
2.2. Test of Swelling Inhibition
2.3. Performance in Water-Based Drilling Fluids
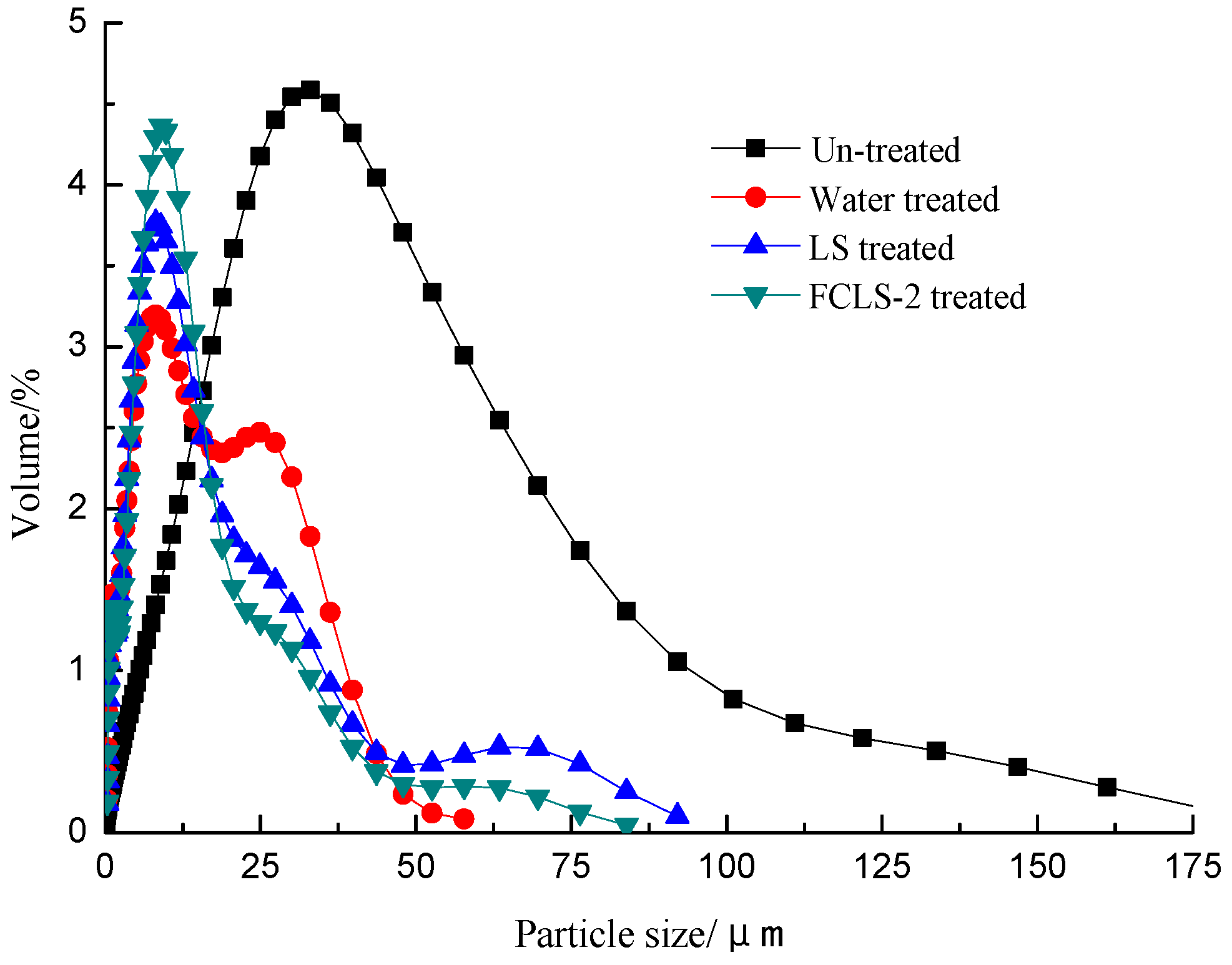
2.4. Particle Size Distribution Test
2.5. TGA
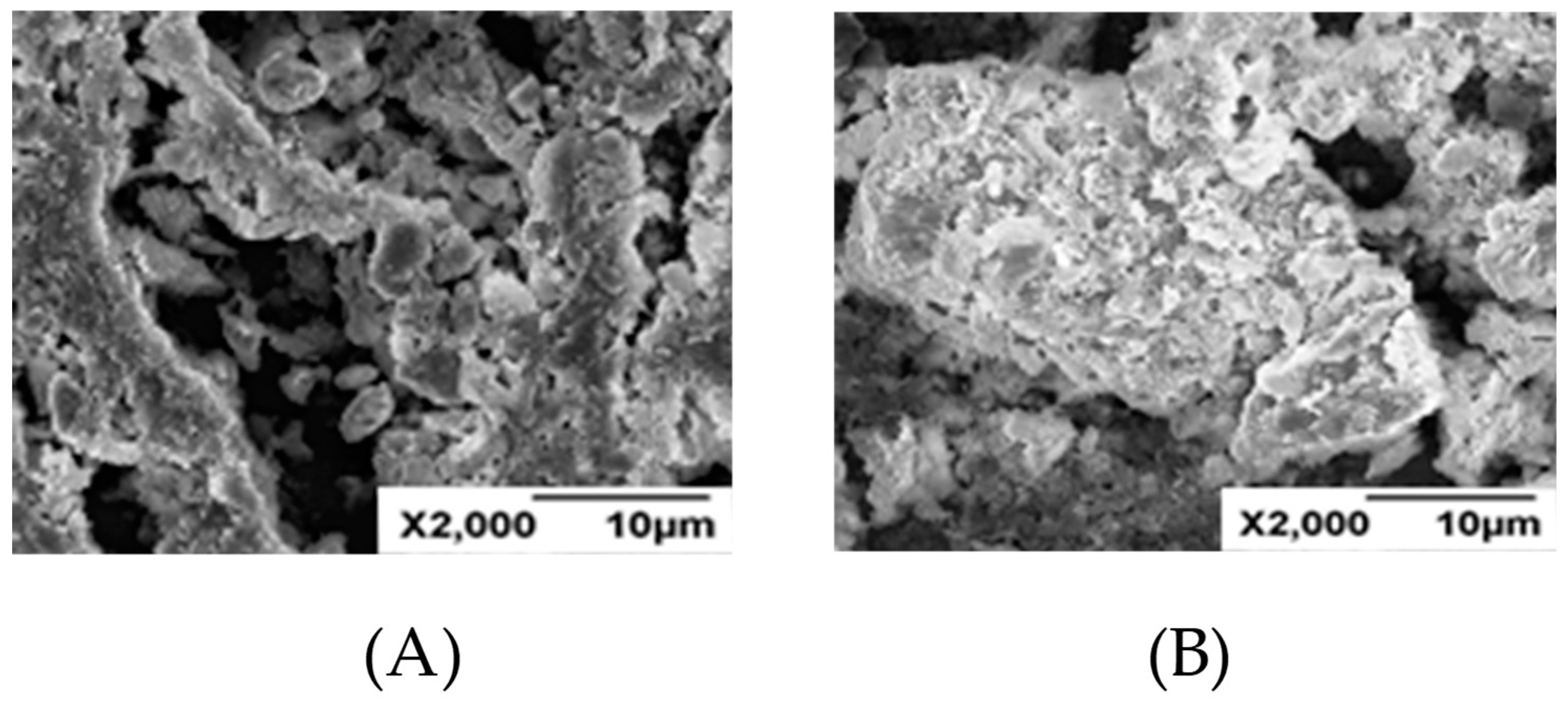
2.6. SEM
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of FCLS
3.3. Evaluation of Inhibitive Ability
3.4. Performance in Drilling Fluid
3.5. Particle Distribution Measurement
3.6. TGA and SEM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.; Yan, J.; Li, L.L.; Zhang, J.; Gu, X.F.; Song, H. Preparation and performance of amine-tartaric salt as potential clay swelling inhibitor. Appl. Clay Sci. 2017, 138, 12–16. [Google Scholar] [CrossRef]
- Muhammad, S.K.; Abdullah, S.S.; Usamah, A.A.; Ibnelwaleed, A.H. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polymer Rev. 2015, 55, 491–530. [Google Scholar]
- Gu, X.F.; Zhang, J.J.; Zhang, J.; Chen, G.; Ma, C.; Zhang, Z.F. Stabilization of montmorillonite by ammoniated lignosulfonates and its use in water-based drilling fluid. Sci. Adv. Mater. 2017, 9, 928–933. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Yang, N.W.; Ma, Y.F. The evaluation of sodium hydroxymethyl lignosulfonate as an ecofriendly drilling fluid additive. Pet. Sci. Technol. 2014, 32, 1816–1823. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Yang, N.W.; Wang, Y.G. Preparation of nitration-oxidation lignosulfonate as an ecofriendly drilling fluid additive. Pet. Sci. Technol. 2014, 32, 1661–1668. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M.A. Polymeric and low molecular weight shale inhibitors: A review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Cheng, C.; Sun, Y.; Hu, Q.; Qu, C.T.; Dong, S.B. Synergistic effect of surfactant and alkali on the treatment of oil sludge. J. Pet. Sci. Eng. 2019, 183, 106420. [Google Scholar] [CrossRef]
- Wu, J.K.; Xu, H.Y.; Xie, Y.N.; Zhou, X.Y. A research and application of strong inhibition drilling fluid. Appl. Mech. Mater. 2014, 675, 1481–1484. [Google Scholar] [CrossRef]
- Yin, X.C.; Zhang, L.M.; Li, Z.M. Studies on new ampholytic cellulose derivative as clay-hydration inhibitor in oil field drilling fluid. J. Appl. Polym. Sci. 1998, 70, 921–926. [Google Scholar] [CrossRef]
- Han, Y.Z.; Song, Z.Q.; Huang, W.A.; Cao, J. Shale inhibitive properties of polyether diamine in water-based drilling fluid. J. Pet. Sci. Technol. 2011, 78, 510–515. [Google Scholar]
- Xi, Y.F.; Ding, Z. Structure of organoclays—an X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yan, Q.B.; Guo, G.; Guo, Z.; Zhang, J.; Chen, G.; Deng, Q. Investigation of oleate graft copolyammonium as a clay swelling inhibitor for shale oil/gas exploration. Petrol. Chem. 2018, 58, 255–259. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Ye, Z.B.; Huang, L.; Bai, Y.; Li, L.L.; Zhang, J.; Qu, C.T.; Chen, G. Preparation and application of a new cross-linked polyammonium as shale inhibitor. J. Appl. Biomater. Funct. Mater. 2018, 16, 119–124. [Google Scholar]
- Zhang, J.; Hu, W.M.; Zhang, L.; Li, T.H.; Cai, D.; Chen, G. Investigation of ammonium-lauric salt as shale swelling inhibitor and a mechanism study. Adsorpt. Sci. Technol. 2019, 37, 49–60. [Google Scholar] [CrossRef]
- Gao, B.Y.; Sun, X.; Yue, Q.Y.; Zhang, D.H. The structure of epichlorohydrin-dimethylamine polymer flocculants with different modifying agents and their properties for decoloration of wastewater. Acta Sci. Circumstantiae 2006, 26, 1977–1982. [Google Scholar]
- Zhang, L.; Li, T.H.; Huang, L.; Qin, Y.Z.; Bin, Y.Z.; Yan, X.; Li, L.L.; Deng, Q. Preparation and application of melamine cross-linked poly ammonium as shale inhibitor. Chem. Central J. 2018, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Zhang, J.; Chen, G. Study on the effect of carboxy-amines small molecule shale inhibitor. Oilfield Chem. 2014, 31, 5. [Google Scholar]
- Zhang, J.; Zhang, Q.; Chen, G. Mechanism study and application of modified polysaccharides as drilling fluid additive with high inhabitability. Technol. Dev. Chem. Ind. 2013, 42, 1–5. [Google Scholar]
- Chen, G.; Gao, L.; Dong, K.; Sun, Y.; Hu, W.M. A green shale inhibitor developed from lignin sulfonate and a mechanism study. J. Biobased. Mater. Bio. 2019, 13, 778–783. [Google Scholar]
- Petroleum, A.P.I. Petroleum and Natural Gas Industries for Field Testing of Drilling Fluids. Available online: https://www.iso.org/standard/41939.html (accessed on 15 June 2001).
Sample Availability: Samples of the Ferric Chloride-Lignin Sulfonates are available from the authors. |



| Name | Mass Ratio | Swelling Rate (%) (60 Min) |
|---|---|---|
| LS | / | 52 |
| FC | / | 65 |
| FCLS-1 | 2:1 | 54 |
| FCLS-2 | 4:1 | 42 |
| FCLS-3 | 6:1 | 44 |
| FCLS-4 | 8:1 | 44 |
| Additives | T/°C | AV | PV | YP | YP/PV | FL | tg |
|---|---|---|---|---|---|---|---|
| /(mPa·s) | /(mPa·s) | /Pa | Pa/(mPa·s) | /mL | |||
| Blank | 25 | 3.6 | 2.1 | 1.40 | 0.67 | 12.0 | 0.07 |
| 120 | 2.6 | 1.8 | 0.70 | 0.39 | 14.0 | 0.18 | |
| PAM | 25 | 8.0 | 4.2 | 3.88 | 0.92 | 13.0 | 0.03 |
| 120 | 7.8 | 4.8 | 3.30 | 0.74 | 15.7 | 0.03 | |
| PAM +0.3%FCLS-3 | 25 | 3.6 | 3.1 | 0.51 | 0.16 | 16.2 | 0.03 |
| 120 | 6.0 | 4.5 | 1.20 | 0.25 | 15.2 | 0.04 | |
| Modified starch | 25 | 4.9 | 2.8 | 2.15 | 0.77 | 11.0 | 0.09 |
| 120 | 9.8 | 8.5 | 2.50 | 0.15 | 15.7 | 0.11 | |
| Modified starch +0.3%FCLS-3 | 25 | 3.5 | 2.2 | 1.33 | 0.60 | 10.1 | 0.19 |
| 120 | 5.7 | 4.5 | 1.20 | 0.55 | 15.5 | 0.11 |
| Additives (180 °C) | Mean/μm | Median/μm |
|---|---|---|
| Un-treated | 38 | 27 |
| Water treated | 8 | 5 |
| 0.3%LS | 8 | 6 |
| 0.3%FCLS-2 | 10 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Gao, L.; Duan, W.; Hu, W.; Du, W.; Gu, X.; Zhang, J.; Chen, G. The Application of Ferric Chloride-Lignin Sulfonate as Shale Inhibitor in Water-Based Drilling Fluid. Molecules 2019, 24, 4331. https://doi.org/10.3390/molecules24234331
Zhang R, Gao L, Duan W, Hu W, Du W, Gu X, Zhang J, Chen G. The Application of Ferric Chloride-Lignin Sulfonate as Shale Inhibitor in Water-Based Drilling Fluid. Molecules. 2019; 24(23):4331. https://doi.org/10.3390/molecules24234331
Chicago/Turabian StyleZhang, Rongjun, Long Gao, Wenguang Duan, Weimin Hu, Weichao Du, Xuefan Gu, Jie Zhang, and Gang Chen. 2019. "The Application of Ferric Chloride-Lignin Sulfonate as Shale Inhibitor in Water-Based Drilling Fluid" Molecules 24, no. 23: 4331. https://doi.org/10.3390/molecules24234331






