Rice Husk Hydrolytic Lignin Transformation in Carbonization Process
Abstract
:1. Introduction
2. Results
2.1. Yield and Composition of Hydrolytic Lignin and Products of its Carbonization
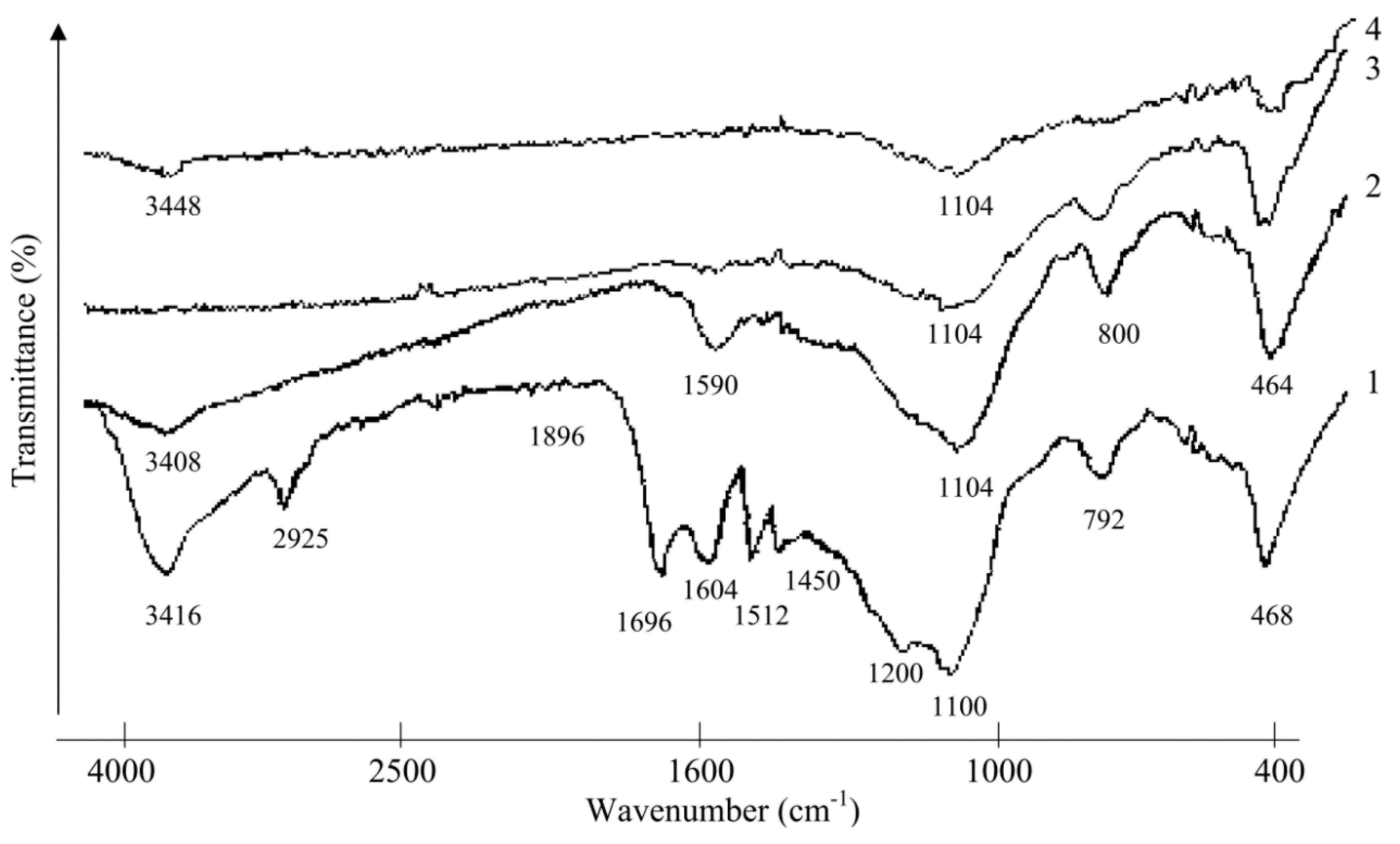
2.2. Investigation of Rice Husk Hydrolytic Lignin Structure and its Carbonization Products by Infrared Spectroscopy
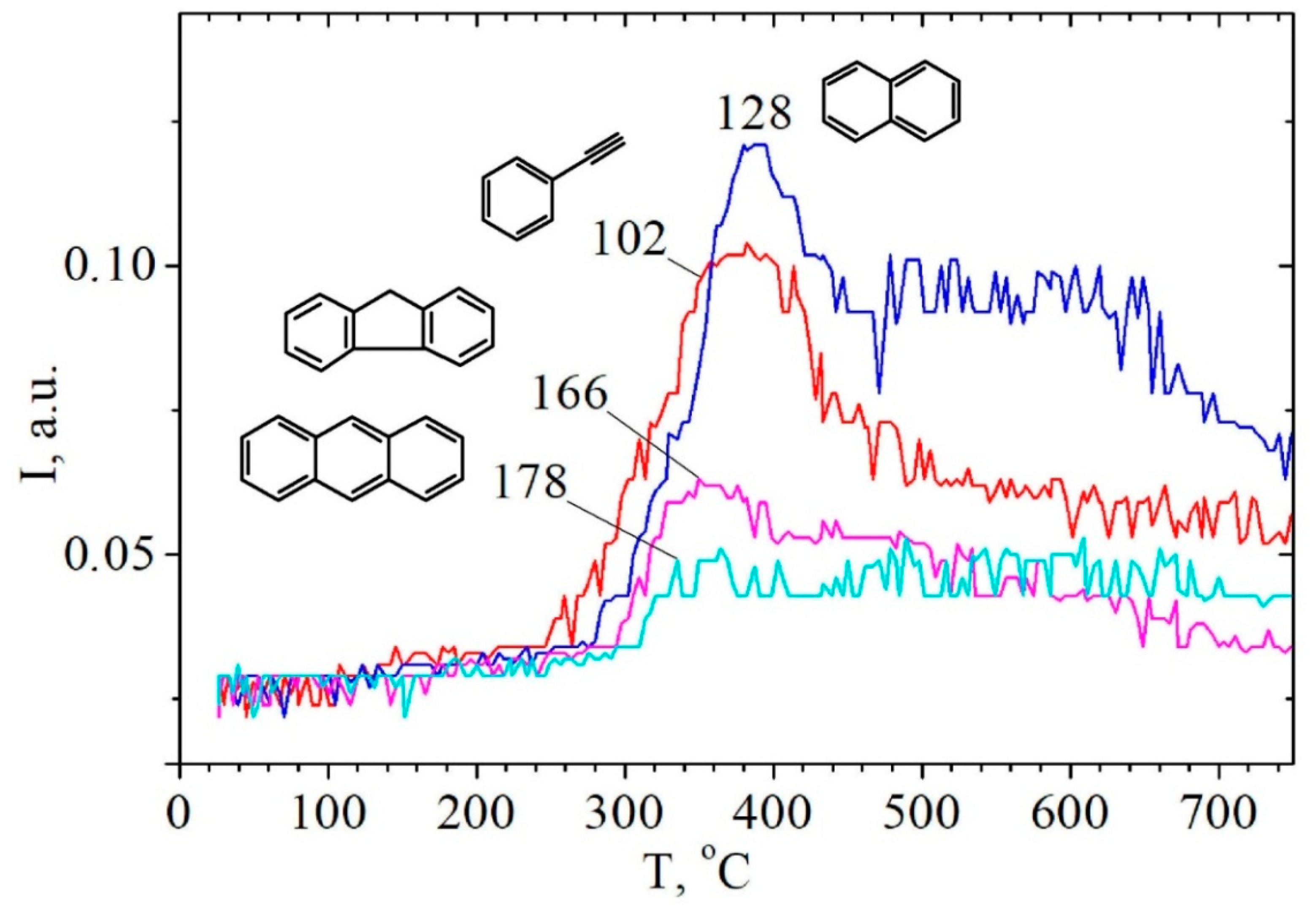
2.3. Study of Hydrolytic Lignin Transformation in the Carbonization Process by Temperature-Programmed Desorption Mass Spectrometry Method
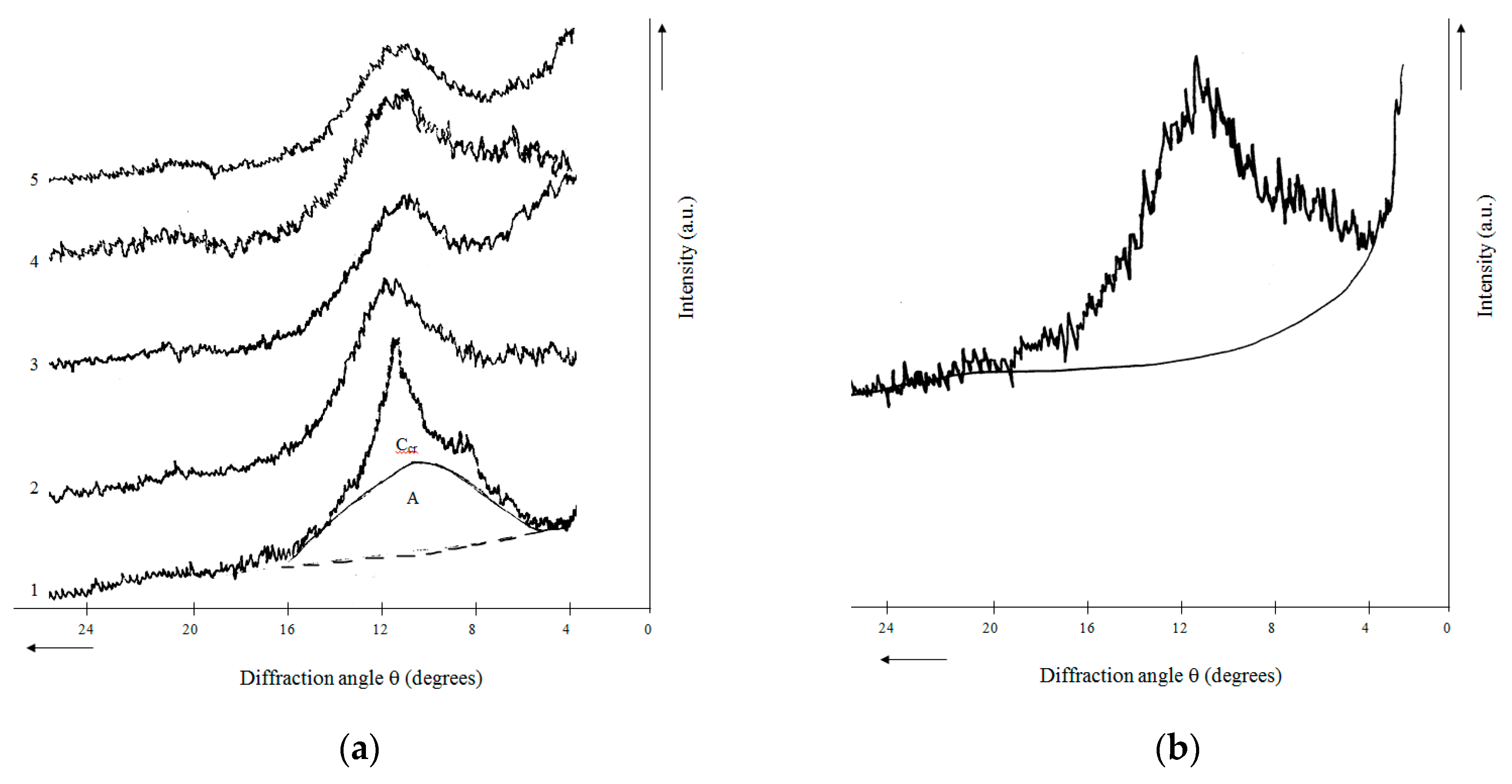
2.4. X-ray Phase Composition and X-ray Diffraction Characteristics of Rice Husk Hydrolytic Lignin and Its Carbonization Products
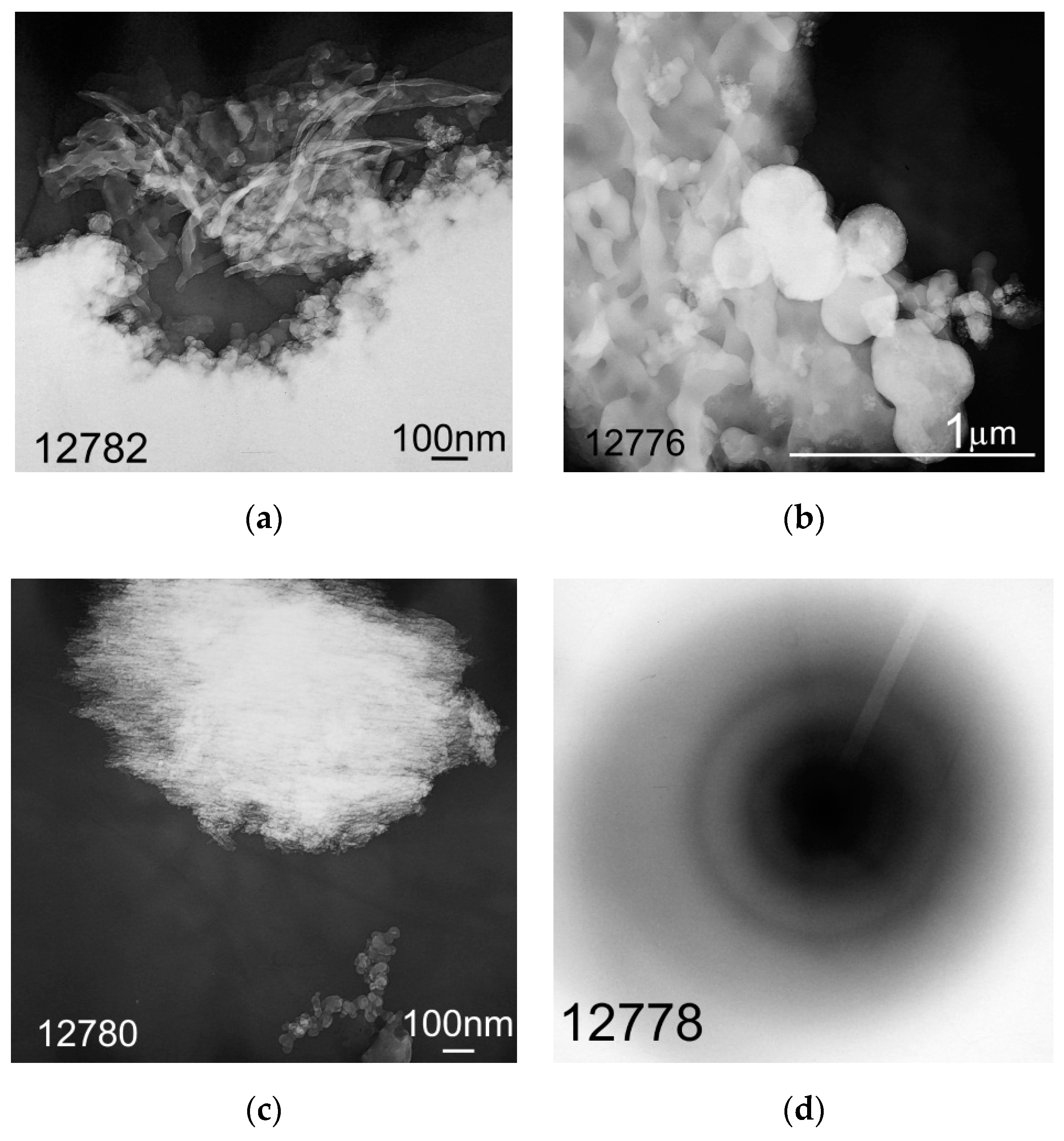
2.5. Study of the Morphology of Hydrolytic Lignin Particles and its Carbonization Products by Transmission Electron Microscopy
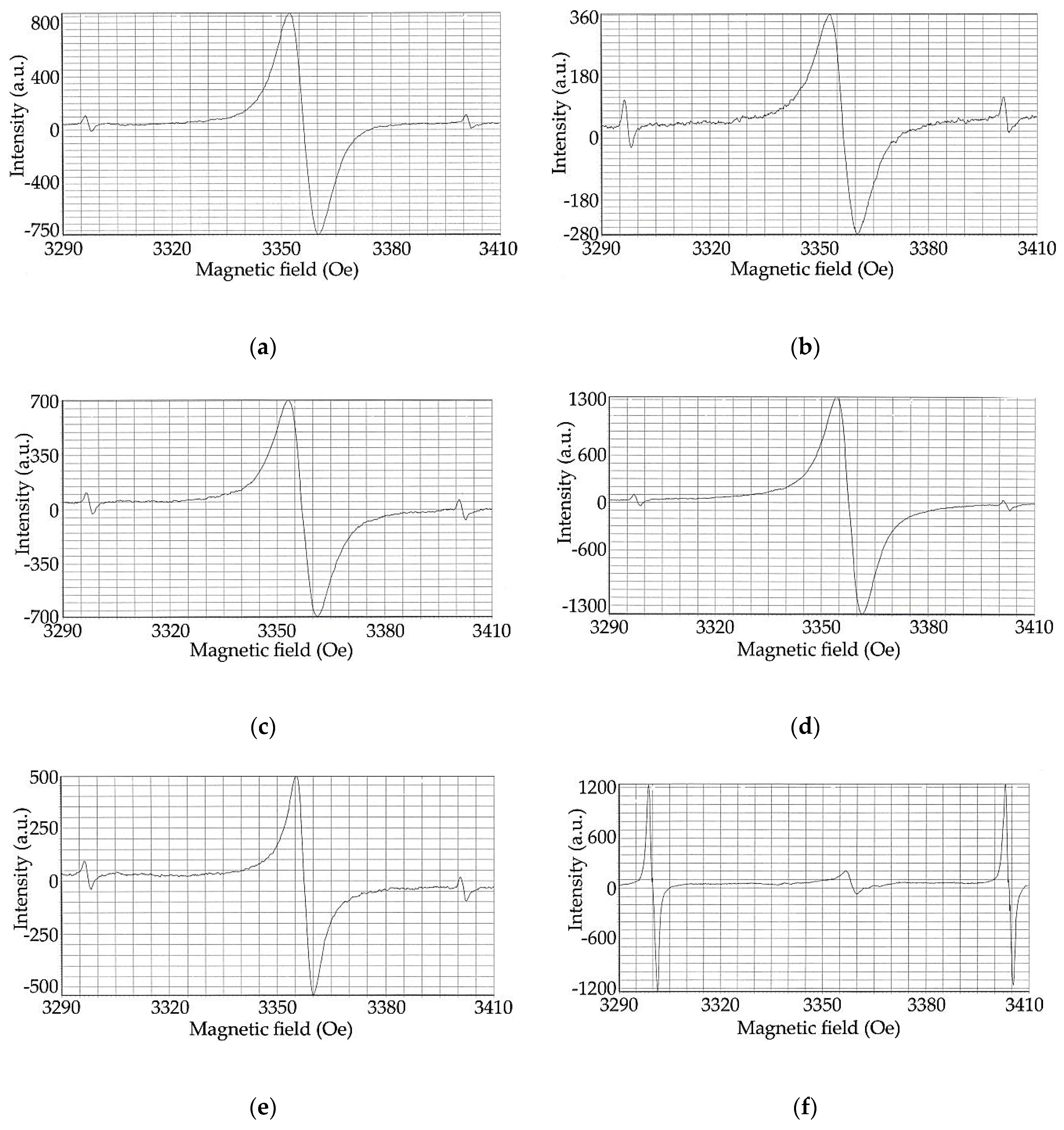
2.6. Investigation of Structural Changes of Hydrolytic Lignin During Carbonization by EPR Spectroscopy Method
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Rice Husk Characteristics and Pretreatment
4.1.2. Hydrolytic Lignin Preparation and Carbonization Processes
4.2. Analytical Methods
4.2.1. Elemental Analysis
4.2.2. Infrared Spectroscopy
4.2.3. Temperature-Programmed Desorption Mass Spectrometry
4.2.4. X-ray Diffraction Analysis
4.2.5. Transmission Electron Microscopy
4.2.6. EPR-Spectroscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, G.; Zou, Q.; Wang, S.; Zhao, Y.; Zhu, L.; Huang, Q. Effect of Torrefaction on the Structure and Pyrolysis Behavior of Lignin. Energy Fuels 2017, 32, 4160–4166. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Yoo, C.G. Editorial: Advancements in Biomass Recalcitrance: The Use of Lignin for the Production of Fuels and Chemicals. Front. Energy Res. 2018, 6, 118. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crop. Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Isroi; Ishola, M.M.; Millati, R.; Syamsiah, S.; Cahyanto, M.N.; Niklasson, C.; Taherzadeh, M.J. Isroi Structural Changes of Oil Palm Empty Fruit Bunch (OPEFB) after Fungal and Phosphoric Acid Pretreatment. Molecules 2012, 17, 14995–15012. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Karnaouri, A.; Cwirzen, A.; Rova, U.; Christakopoulos, P. Formation of Lignin Nanoparticles by Combining Organosolv Pretreatment of Birch Biomass and Homogenization Processes. Molecules 2018, 23, 1822. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.; El Mansouri, N.E.; Ferrando, F.; Salvadó, J. All-lignocellulosic Fiberboard from Steam Exploded Arundo Donax, L. Molecules 2018, 23, 2088. [Google Scholar] [CrossRef]
- Lagerquist, L.; Pranovich, A.; Sumerskii, I.; Von Schoultz, S.; Vähäsalo, L.; Willför, S.; Eklund, P. Structural and Thermal Analysis of Softwood Lignins from a Pressurized Hot Water Extraction Biorefinery Process and Modified Derivatives. Molecules 2019, 24, 335. [Google Scholar] [CrossRef]
- Zinovyev, G.; Sumerskii, I.; Rosenau, T.; Balakshin, M.; Potthast, A. Ball milling’s effect on pine milled wood lignin’s structure and molar mass. Molecules 2018, 23, 2223. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Sevastyanova, O. Carbon Materials from Technical Lignins: Recent Advances. Top. Curr. Chem. 2018, 376, 33. [Google Scholar] [CrossRef]
- Nikitin, V.M.; Obolenskaya, A.V.; Shegolev, V.P. Wood and Cellulose Chemistry; Lesnaya Promyshlennost: Moscow, Russia, 1978. [Google Scholar]
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin—An alternative precursor for sustainable and cost-effective automotive carbon fiber. J. Mater. Res. Technol. 2015, 4, 283–296. [Google Scholar] [CrossRef]
- Efremova, S.V.; Korolev, Y.M.; Sukharnikov, Y.I. X-ray diffraction characterization of silicon-carbon nanocomposites produced from rice husk and its derivatives. Dokl. Chem. 2008, 419, 78–81. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.; Ribeiro Carrott, M.M. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Efremova, S.V. Rice hull as a renewable raw material and its processing routes. Russ. J. Gen. Chem. 2012, 82, 999–1005. [Google Scholar] [CrossRef]
- Zharmenov, A.; Yefremova, S.; Sukharnikov, Y.; Bunchuk, L.; Kablanbekov, A.; Anarbekov, K.; Murtazayeva, D.; Yessengarayev, Y. Carbonaceous materials from rice husk: Production and application in industry and agriculture. Inz. Miner. J. Pol. Miner. Eng. Soc. 2018, 1, 263–274. [Google Scholar] [CrossRef]
- Yefremova, S.; Sukharnikov, Y.; Terlikbayeva, A.; Zharmenov, A.; Bunchuk, L.; Anarbekov, K.; Shabanova, T. Influence of vegetable polymers´ nature on carbon materials structure formation. In Mine Planning and Equipment Selection; Springer International Publishing AG: Cham, Switzerland, 2014; pp. 1107–1115. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Su, S.; Wang, J. Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrolysis 2012, 98, 177–183. [Google Scholar] [CrossRef]
- Boldyrev, A. Infrared Spectra of Minerals; Nedra: Moscow, Russia, 1976. [Google Scholar]
- Lebedev, A. Mass Spectrometry in Organic Chemistry; Binom: Moscow, Russia, 2003; 493p. [Google Scholar]
- Palyanitsa, B.B.; Kulik, T.V.; Dudik, O.O.; Toncha, O.L.; Cherniavska, T.V. Study of the thermal decomposition of some components of biomass by desorption mass spectrometry. In Proceedings of the International Congress on Energy Efficiency and Energy Related Materials (ENEFM2013), Antalya, Turkey, 9–12 October 2013; Springer International Publishing AG: Cham, Switzerland, 2014; pp. 19–25. [Google Scholar] [CrossRef]
- Kulik, T.V.; Barvinchenko, V.N.; Palyanitsa, B.B.; Smirnova, O.V.; Pogorelyi, V.K.; Chuiko, A.A. A Desorption mass spectrometry study of the interaction of cinnamic acid with a silica surface. Russ. J. Phys. Chem. 2007, 81, 83–90. [Google Scholar] [CrossRef]
- Kulik, T.V.; Lipkovska, N.A.; Barvinchenko, V.N.; Palyanytsa, B.B.; Kazakova, O.A.; Dovbiy, O.A.; Pogorelyi, V.K. Interactions between bioactive ferulic acid and fumed silica by UV-VIS spectroscopy, FT-IR, TPD MS investigation and quantum chemical methods. J. Colloid Interface Sci. 2009, 339, 60–68. [Google Scholar] [CrossRef]
- Kulik, T.V.; Lipkovska, N.A.; Barvinchenko, V.N.; Palyanytsa, B.B.; Kazakova, O.A.; Dudik, O.O.; Menyhárd, A.; László, K. Thermal transformation of bioactive caffeic acid on fumed silica seen by UV-VIS spectroscopy, thermogravimetric analysis, temperature programmed desorption mass spectrometry and quantum chemical methods. J. Colloid Interface Sci. 2016, 470, 132–141. [Google Scholar] [CrossRef]
- Nastasiienko, N.; Palianytsia, B.; Kartel, M.; Larsson, M.; Kulik, T. Thermal transformation of caffeic acid on the nanosized cerium dioxide studied by temperature programmed desorption mass-spectrometry, thermogravimetric analysis and FTIR-spectroscopy. Colloids Interfaces 2019, 3, 34. [Google Scholar] [CrossRef]
- Soukup, M.; Martinka, M.; Bosnić, D.; Čaplovičová, M.; Elbaum, R.; Lux, A. Formation of silica aggregates in sorghum root endodermis is predetermined by cell wall architecture and development. Ann. Bot. 2017, 120, 739–753. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L. Infra-Red Spectra of Complex Molecule; Izdatelstvo inostrannoj literatury: Moscow, Russia, 1963; p. 592. [Google Scholar]
- Kulyk, K.; Palianytsia, B.; Alexander, J.D.; Azizova, L.; Borysenko, M.; Kartel, M.; Larsson, M.; Kulik, T. Kinetics of valeric acid ketonization and ketenization in catalytic pyrolysis on nanosized SiO2, γ-Al2O3, CeO2/SiO2, Al2O3/SiO2 and TiO2/SiO2. ChemPhysChem 2017, 18, 1943–1955. [Google Scholar] [CrossRef]
- Kulyk, K.S.; Ishchenko, V.V.; Palyanytsya, B.B.; Khylya, V.P.; Borysenko, M.V.; Kulyk, T.V. A TPD-MS study of the interaction of coumarins and their heterocyclic derivatives with a surface of fumed silica and nanosized oxides CeO2/SiO2, TiO2/SiO2, Al2O3/SiO2. J. Mass Spectrom. 2010, 45, 750–761. [Google Scholar] [CrossRef]
- Preobrajenskaya, N.A. X-ray crystallography and structure of carbonaceous substances. Khimiya Tverd. Topl. 1992, 5, 93–99. [Google Scholar]
- Gagarin, S.G.; Korolev, Y.M. Transformation of the X-ray phase structure of the organic mass of coal in a series of regional metamorphism (review). Koks Khimiya 1994, 11, 2–6. [Google Scholar]
- Korolev, Y.M. X-ray of amorphous carbonaceous substances. Ph.D. Thesis, Institute of Chemical Physics, USSR Academy of Sciences, Moscow, Russia, 30 April 1991. [Google Scholar]
- Kholkin, Y.I.; Stepovaya, L.P.; Mochalina, M.D. Study of radiation and thermal destruction of woody polysaccharides by EPR and IR spectroscopy. Khimiya Drev. 1971, 8, 107–117. [Google Scholar]
- Mansurov, Z.A.; Ryabikin, Y.A.; Zhylybayeva, N.K.; Zashkvara, O.Y.; Mansurova, R.M. Study of the possibility of nanostructures formation in the process of apricot kernel carbonization by the EPR method. In Proceedings of the 2nd International Symposium Physics and Chemistry of Carbon Materials, Almaty, Kazakhstan, 18–20 September 2002; pp. 273–275. [Google Scholar]
- Yefremova, S.V. Sukharnikov Yu. I. Study of nanostructure and phase composition of silicon-carbon fillers produced from the rice hulls for elastomers. Ann. Univ. Mariae Curie-Skłodowska Sect. Chem. 2007, 62, 194–209. [Google Scholar]
- Yefremova, S.V.; Korolev, Y.M.; Sukharnikov, Y.I.; Kablanbekov, A.A.; Anarbekov, K.K. Structural transformations of carbon materials in the processes of preparation from plant raw materials. Solid Fuel Chem. 2016, 50, 152–157. [Google Scholar] [CrossRef]
- Yefremova, S.V.; Sukharnikov, Y.I.; Anderson Corby, G. Integrated processing of the rice hulls with producing materials of polyfunctional purpose. J Solid Waste Technol. Manag. 2009, 2, 749–756. [Google Scholar]
- Kondratiyev, I.A.; Lavrukhin, C.P.; Rossiyskiy, V.A.; Zlatkis, A.M.; Sukharnikov, Y.I. Self-Lubricated Carbon Material. RU Patent 2,101,261, 10 January 1998. [Google Scholar]
- Obolenskaya, A.V.; Shegolev, V.P.; Akim, G.L. Practical Work in the Chemistry of Wood and Pulp; Lesnaya Promyshlennost: Moscow, Russia, 1965; p. 412. [Google Scholar]
- Kulik, T.V. Use of TPD-MS and linear free energy relationships for assessing the reactivity of aliphatic carboxylic acids on a silica surface. J. Phys. Chem. C 2012, 116, 570–580. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Sample | Content, Mass % | |||
|---|---|---|---|---|
| C | H | N + Oorg | Mineral Residue | |
| L-500 (OSG) | 51.85 | 2.64 | 4.74 | 40.77 |
| L-650 (OSG) | 51.04 | 2.65 | 5.53 | 40.78 |
| L-1000 (OSG) | 49.80 | 2.65 | 5.79 | 41.76 |
| L-500 (10–20 kPa) | 44.31 | 2.04 | 3.65 | 50.00 |
| L-650 (10–20 kPa) | 42.2 | 2.00 | 3.20 | 52.60 |
| L-1000 (10–20 kPa) | 40,.54 | 1.36 | 3.00 | 55.10 |
| Pyrolysis Product or its Fragment Ion | m/z1 | I, a.u. | Trange, °C | Tmax, °C | n | E 1, kJ/mol | ν0, s−1 | dS 1, cal/(K × mol) | R22 |
|---|---|---|---|---|---|---|---|---|---|
| Phenols | |||||||||
| Tropyliumion, C7H7+ | 91 | 1063 | 220–570 | 370 | - | - | - | - | - |
| Phenol | 94 | 0.840 | 220–600 | 355 | - | - | - | - | - |
| Cresols | 107 | 1035 | 240–530 | 370 | - | - | - | - | - |
| Pyrocatechol | 110 | 0.817 | 250–520 | 384 | 1 | 113 | 2.23 × 106 | −31 | 0.931 |
| Guaiacol | 124 | 0.633 | 250–500 | 340 | - | - | - | - | - |
| Syringol | 154 | 0.092 | 270–470 | 360 | - | - | - | - | - |
| Methylguaiacol | 138 | 0.646 | 250–430 | 330 | 1 | 142 | 1.02 × 1010 | −14 | 0.947 |
| 4-Vinylphenols | |||||||||
| 4-Vinylphenol | 120 | 0.355 | 210–470 | 325 | 1 | 77 | 1.17 × 104 | −41 | 0.941 |
| 4-Vinylpyrocatechol | 136 | 0.144 | 260–430 | 355 | 1 | 104 | 1.17 × 106 | −24 | 0.939 |
| 4-Vinylguaiacol | 150 | 0.257 | 235–400 | 320 | 1 | 107 | 9.56 × 106 | −28 | 0.970 |
| 4-Vinyl-methylguaiacol | 164 | 0.109 | 260–420 | 335 | 1 | 100 | 1.38 × 106 | −32 | 0.952 |
| Aromatic/Polycyclic aromatic hydrocarbons | |||||||||
| Benzene | 78 | 0.761 | 250–750… | 390 | - | - | - | - | - |
| Phenylacetylene | 102 | 0.104 | 250–750… | 380 | - | - | - | - | - |
| Naphthalene | 128 | 0.121 | 270–750… | 390 | - | - | - | - | - |
| 9H-Fluorene | 166 | 0.063 | 300–700 | 365 | - | - | - | - | - |
| Anthracene or Phenanthrene | 178 | 0.053 | 310–750… | - | - | - | - | - | - |
| Sample | Composition of Carbon-Containing Component, % | Parameters G | ||||
|---|---|---|---|---|---|---|
| G | N | H | d002, nm | Lc, nm | La, nm | |
| L-400 | - | 80 | 20 | - | - | - |
| L-500 | 44 | 41 | 15 | 0.38 | 2.1 | - |
| L-650 | 43 | 35 | 22 | 0.381 | 4.0 | - |
| L-800 | 44 | 38 | 18 | 0.375 | 4.6 | - |
| L-1000 | 62 | - | 38 | 0.375 | 4.7 | - |
| Sample | PMCs Concentration, spin g−1 | EPR Line Width | g-Factor |
|---|---|---|---|
| L | 7.7 · 10 16 | 6.3 | 2.0032 |
| L-300 | 3.6 · 10 16 | 6.3 | 2.0030 |
| L-450 | 6.5 · 10 16 | 6.7 | 2.0030 |
| L-550 | 3.1 · 10 17 | 6.2 | 2.0028 |
| L-650 | 1.6 · 10 17 | 3.8 | 2.0027 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yefremova, S.; Zharmenov, A.; Sukharnikov, Y.; Bunchuk, L.; Kablanbekov, A.; Anarbekov, K.; Kulik, T.; Nikolaichuk, A.; Palianytsia, B. Rice Husk Hydrolytic Lignin Transformation in Carbonization Process. Molecules 2019, 24, 3075. https://doi.org/10.3390/molecules24173075
Yefremova S, Zharmenov A, Sukharnikov Y, Bunchuk L, Kablanbekov A, Anarbekov K, Kulik T, Nikolaichuk A, Palianytsia B. Rice Husk Hydrolytic Lignin Transformation in Carbonization Process. Molecules. 2019; 24(17):3075. https://doi.org/10.3390/molecules24173075
Chicago/Turabian StyleYefremova, Svetlana, Abdurassul Zharmenov, Yurij Sukharnikov, Lara Bunchuk, Askhat Kablanbekov, Kuanish Anarbekov, Tetiana Kulik, Alina Nikolaichuk, and Borys Palianytsia. 2019. "Rice Husk Hydrolytic Lignin Transformation in Carbonization Process" Molecules 24, no. 17: 3075. https://doi.org/10.3390/molecules24173075






