Generation of Quality Hit Matter for Successful Drug Discovery Projects
Acknowledgments
Conflicts of Interest
References
- Pandey, P.; Roy, K.K.; Liu, H.; Ma, G.; Pettaway, S.; Alsharif, W.F.; Gadepalli, R.S.; Rimoldi, J.M.; McCurdy, C.R.; Cutler, S.J.; et al. Structure-Based Identification of Potent Natural Product Chemotypes as Cannabinoid Receptor 1 Inverse Agonists. Molecules 2018, 23, 2630. [Google Scholar] [CrossRef]
- Salomatina, O.V.; Popadyuk, I.I.; Zakharenko, A.L.; Zakharova, O.D.; Fadeev, D.S.; Komarova, N.I.; Reynisson, J.; Arabshahi, H.J.; Chand, R.; Volcho, K.P.; et al. Novel Semisynthetic Derivatives of Bile Acids as Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Molecules 2018, 23, 679. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zheng, Z.; Zhang, S.; Zheng, X.; Meng, F.; Yuan, J.; Xu, Y.; Huang, C. Fragment-Based Lead Generation of 5-Phenyl-1H-pyrazole-3-carboxamide Derivatives as Leads for Potent Factor Xia Inhibitors. Molecules 2018, 23, 2002. [Google Scholar] [CrossRef] [PubMed]
- Saydmohammed, M.; Vollmer, L.L.; Onuoha, E.O.; Maskrey, T.; Gibson, G.; Watkins, S.C.; Wipf, P.; Vogt, A.; Tsang, M. A High-Content Screen Reveals New Small-Molecule Enhancers of Ras/Mapk Signaling as Probes for Zebrafish Heart Development. Molecules 2018, 23, 1691. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; El-Houri, R.B. Development of an In Vitro Screening Platform for the Identification of Partial PPARγ Agonists as a Source for Antidiabetic Lead Compounds. Molecules 2018, 23, 2431. [Google Scholar] [CrossRef]
- Jiménez, A.; García, P.; de la Puente, S.; Madrona, A.; Camarasa, M.J.; Pérez-Pérez, M.; Quintela, J.; Portillo, F.P.; San-Félix, A. A Novel Class of Cationic and Non-Peptidic Small Molecules as Hits for the Development of Antimicrobial Agents. Molecules 2018, 23, 1513. [Google Scholar]
- Higashino, Y.; Okamoto, T.; Mori, K.; Kawasaki, T.; Hamada, M.; Nakajima, N.; Saito, A. Regioselective Synthesis of Procyanidin B6, A 4-6-Condensed (+)-Catechin Dimer, by Intramolecular Condensation. Molecules 2018, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Pilkington, L.I.; Natalie, A.; Haverkate, N.A.; van Rensburg, M.; Leung, E.; Kumara, S.; Denny, W.A.; Barker, D.; Alsuraifi, A.; et al. Investigation into Improving the Aqueous Solubility of the Thieno[2,3-b]pyridine Anti-Proliferative Agents. Molecules 2018, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Sari, S.; Leung, E.; Pilkington, L.I.; van Rensburg, M.; Barker, D.; Reynisson, J. GPCR Modulation of Thieno[2,3-b]pyridine Anti-Proliferative Agents. Molecules 2017, 22, 2254. [Google Scholar] [CrossRef]
- Opassi, G.; Gesù, A.; Massarotti, A. The hitchhiker’s guide to the chemical-biological galaxy. Drug Dis. Today 2018, 23, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Siramshetty, V.B.; Preissner, R. Drugs as habitable planets in the space of dark chemical matter. Drug Dis. Today 2018, 23, 481–486. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Beall, J.B.; Walters, M.A. Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Beall, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 2010, 2719–2740. [Google Scholar] [CrossRef]
- Eurtivong, C.; Reynisson, J. The Development of a Weighted Index to Optimise Compound Libraries for High Throughput Screening. Mol. Inf. 2018, 37, 1800068. [Google Scholar] [CrossRef]
- Kim, E.J.; Matuszek, A.M.; Yu, B.; Reynisson, J. Theoretical investigations into the role of aryl nitrenium ions’ stability on their mutagenic potential. Aust. J. Chem. 2011, 67, 910–915. [Google Scholar] [CrossRef]
- Ren, L.; Reynisson, J.; Perera, C.O.; Hemar, Y. The physicochemical properties of a new class of anticancer fungalpolysaccharides: A comparative study. Carbohydr. Polym. 2013, 97, 177–187. [Google Scholar] [CrossRef]
- Zhu, F.; Logan, G.; Reynisson, J. Wine Compounds as a Source for HTS Screening Collections. A Feasibility Study. Mol. Inf. 2012, 31, 847–855. [Google Scholar] [CrossRef]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. An In-Pharm-ative Educational Poster Anthology Highlighting the Therapeutic Agents That Chronicle Our Medicinal History. J. Chem. Educ. 2013, 90, 1403–1405. [Google Scholar] [CrossRef]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals To Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Smith, B.R.; Eastman, C.M.; Njardarson, J.T. Beyond C, H, O, and N! Analysis of the Elemental Composition of U.S. FDA Approved Drug Architectures. J. Med. Chem. 2014, 57, 9764–9773. [Google Scholar]
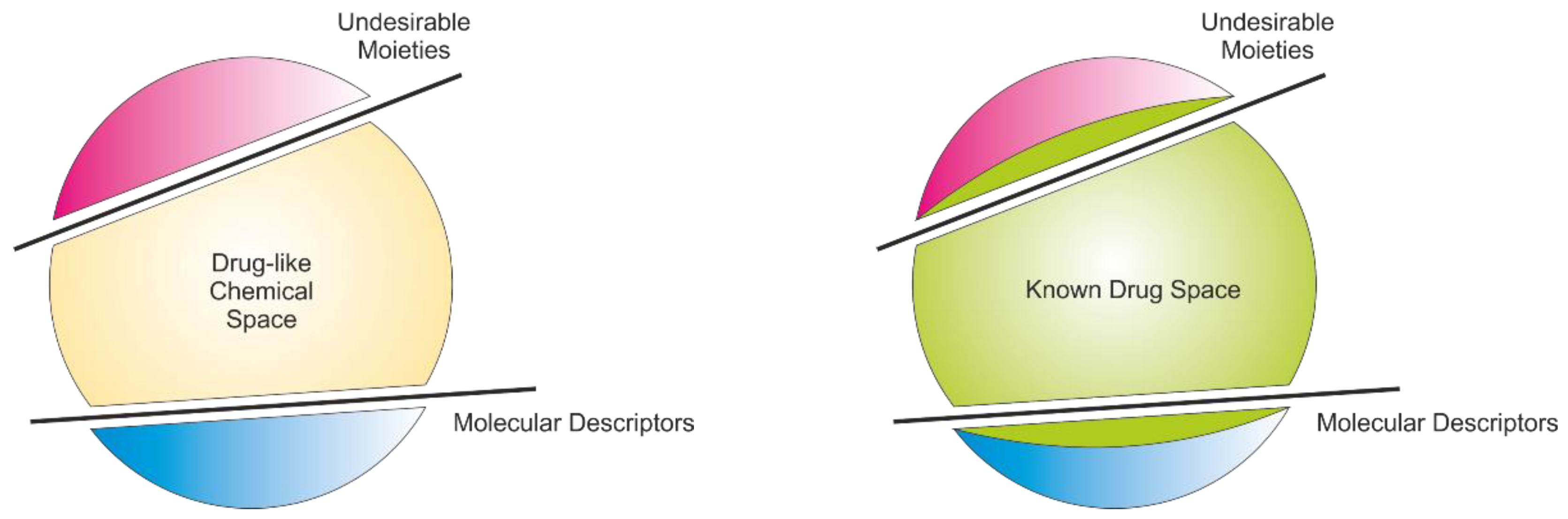
- Mirza, A.; Desai, R.; Reynisson, J. Known Drug Space as a Metric in Determining the Boundaries of Drug-Like Chemical Space. Eur. J. Med. Chem. 2009, 44, 5006–5011. [Google Scholar] [CrossRef]
- Axerio-Cilies, P.; Castañeda, I.P.; Mirza, A.; Reynisson, J. Investigation of the incidence of ‘‘undesirable’’ molecular moieties for high-throughput screening compound libraries in marketed drug compounds. Eur. J. Med. Chem. 2009, 44, 1128–1134. [Google Scholar] [CrossRef]
- Bade, R.; Chan, H.-F.; Reynisson, J. Characteristics of Known Drug Space. Natural Products, their Derivatives and Synthetic Drugs. Eur. J. Med. Chem. 2010, 45, 5646–5652. [Google Scholar] [CrossRef]
- Drew, K.L.M.; Baiman, H.; Khwaounjoo, P.; Yu, B.; Reynisson, J. Size estimation of chemical space: How big is it? J. Pharm. Pharmacol. 2012, 64, 490–495. [Google Scholar] [CrossRef]
- Matuszek, A.M.; Reynisson, J. Defining Known Drug Space Using DFT. Mol. Inf. 2016, 35, 46–53. [Google Scholar] [CrossRef]
- Zafar, A.; Reynisson, J. Hydration Free Energy as a Molecular Descriptor in Drug Design: A Feasibility Study. Mol. Inf. 2016, 35, 207–217. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynisson, J. Generation of Quality Hit Matter for Successful Drug Discovery Projects. Molecules 2019, 24, 381. https://doi.org/10.3390/molecules24030381
Reynisson J. Generation of Quality Hit Matter for Successful Drug Discovery Projects. Molecules. 2019; 24(3):381. https://doi.org/10.3390/molecules24030381
Chicago/Turabian StyleReynisson, Jóhannes. 2019. "Generation of Quality Hit Matter for Successful Drug Discovery Projects" Molecules 24, no. 3: 381. https://doi.org/10.3390/molecules24030381




