Comparison of the Partition Efficiencies of Multiple Phenolic Compounds Contained in Propolis in Different Modes of Acetonitrile–Water-Based Homogenous Liquid–Liquid Extraction
Abstract
:1. Introduction
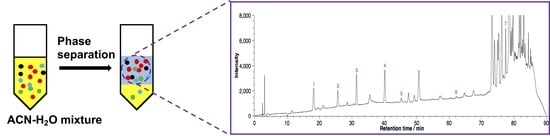
2. Results and Discussion
2.1. Salting-Out Assisted Liquid–Liquid Extraction
2.2. Sugaring-Out Assisted Liquid–Liquid Extraction
2.3. Hydrophobic-Solvent Assisted Liquid–Liquid Extraction
2.4. Subzero-Temperature Assisted Liquid–Liquid Extraction
3. Materials and Methods
3.1. Materials
3.2. Homogeneous Liquid–Liquid Extraction
3.3. HPLC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bijttebier, S.; der Auwera, A.V.; Foubert, K.; Voorspoels, S.; Pieters, L.; Apers, S. Bridging the gap between comprehensive extraction protocols in plant metabolomics studies and method validation. Anal. Chim. Acta 2016, 935, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; dos Santos, D.C.M.; Garcia, A.; Barbas, C. Analytical protocols based on LC-MS.; GC-MS and CE-MS for nontargeted metabolomics of biological tissues. Bioanalysis 2014, 6, 1657–1677. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Trends in analysis of pesticide residues to fulfil the European Regulation (EC) No. 1107/2009. Trends Anal. Chem. 2016, 80, 568–580. [Google Scholar] [CrossRef]
- Herrero, M.; Simó, C.; García-Cañas, V.; Ibáñez, E.; Cifuentes, A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom. Rev. 2012, 31, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Chen, G.L.; Wu, J.L.; Li, N.; Liu, Z.H.; Guo, M.Q. Recent development in mass spectrometry and its hyphenated techniques for the analysis of medicinal plants. Phytochem. Anal. 2018, 29, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Causon, T.J.; Hann, S. Review of sample preparation strategies for MS-based metabolomic studies in industrial biotechnology. Anal. Chim. Acta 2016, 938, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.N.; Ioannou, K.-I.G. Recent developments in homogeneous and dispersive liquid-liquid extraction for inorganic elements determination. A review. Talanta 2009, 80, 413–421. [Google Scholar] [CrossRef]
- Valente, I.M.; Rodrigues, J.A. Recent advances in salt-assisted LLE for analyzing biological samples. Bioanalysis 2015, 7, 2187–2193. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mohebbi, A.; Feriduni, B. Development of a simple and efficient pretreatment technique named pH-dependent continuous homogenous liquid–liquid extraction. Anal. Methods 2016, 8, 5676–5683. [Google Scholar] [CrossRef]
- Wang, B.; Ezejias, T.; Feng, H.; Blaschek, H. Sugaring-out: A novel phase separation and extraction system. Chem. Eng. Sci. 2008, 63, 2595–2600. [Google Scholar] [CrossRef]
- Liu, G.Z.; Zhou, N.Y.; Zhang, M.S.; Li, S.J.; Tian, Q.Q.; Chen, J.T.; Chen, B.; Wu, Y.N.; Yao, S.Z. Hydrophobic solvent induced phase transition extraction to extract drugs from plasma for high performance liquid chromatography-mass spectrometric analysis. J. Chromatogr. A 2010, 1217, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Akane, A. Subzero-temperature liquid-liquid extraction of benzodiazepines for high performance liquid chromatography. Anal. Chem. 1999, 71, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.M.; Moreira, M.M.; Neves, P.; da Fé, T.; Gonçalves, L.M.; Almeida, P.J.; Rodrigues, J.A. An insight on salting-out assisted liquid-liquid extraction for phytoanalysis. Phytochem. Anal. 2017, 28, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, H.; Ezeji, T.; Blaschek, H. Sugaring-out separation of acetonitrile from its aqueous solution. Chem. Eng. Technol. 2008, 31, 1869–1874. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chuang, H.Y.; Chen, H.H.; Wu, Y.W.; Cheng, S.H.; Huang, T.C. Application of sugaring-out extraction for the determination of sulfonamides in honey by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 2010, 1217, 7812–7815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Myasein, F.; Wu, H.Q.; El-Shourbagy, T.A. Sugaring-out assisted liquid/liquid extraction with acetonitrile for bioanalysis using liquid chromatography-mass spectrometry. Microchem. J. 2013, 108, 198–202. [Google Scholar] [CrossRef]
- Cai, B.D.; Ye, E.C.; Yuan, B.F.; Feng, Y.Q. Sequential solvent induced phase transition extraction for profiling of endogenous phytohormones in plants by liquid chromatography-mass spectrometry. J. Chromatogr. B 2015, 1004, 23–29. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, S.S.; Liu, X.Z.; Yuan, F.F.; Liang, Y.H.; Shi, Z.H. Determination of five anthraquinone derivatives in sticky traditional Chinese patent medicines by subzero-temperature liquid-liquid extraction combined with high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 584–590. [Google Scholar] [CrossRef]
- Torbati, M.; Farajzadeh, M.A.; Torbati, M.; Nabil, A.A.A.; Mohebbi, A.; Mogaddam, M.R.A. Development of salt and pH–induced solidified floating organic droplets homogeneous liquid–liquid microextraction for extraction of ten pyrethroid insecticides in fresh fruits and fruit juices followed by gas chromatography mass spectrometry. Talanta 2018, 176, 565–572. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mohebbi, A.; Mogaddam, M.R.A.; Davaran, M.; Norouzi, M. Development of salt-induced homogenous liquid-liquid microextraction based on iso-propanol/sodium sulfate system for extraction of some pesticides in fruit juices. Food Anal. Methods 2018, 11, 2497–2507. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Fernandes-Silva, C.C.; Gighi, A.A.; Salatino, M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC–MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and functional characterization of Italian propolis obtained by different harvesting methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Wu, Z.S.; Wang, Z.Y.; Zhang, H.C. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid-Based Compl. Alt. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, N.; Leo, C.P. Microwave-assisted extraction of Trigona propolis: The effects of processing parameters. Int. J. Food Eng. 2015, 11, 861–870. [Google Scholar] [CrossRef]
- ChemSpider. Available online: http://www.chemspider.com (accessed on 3 January 2018).
- Valente, I.M.; Goncalves, L.M.; Rodrigues, J.A. Another glimpse over the salting-out assisted liquid-liquid extraction in acetonitrile/water mixtures. J. Chromatogr. A 2013, 1308, 58–62. [Google Scholar] [CrossRef]
- Tu, X.J.; Sun, F.Y.; Wu, S.Y.; Liu, W.Y.; Gao, Z.S.; Huang, S.K.; Chen, W.B. Comparison of salting-out and sugaring-out liquid-liquid extraction methods for the partition of 10-hydroxy-2-decenoic acid in royal jelly and their co-extracted protein content. J. Chromatogr. B 2018, 1073, 90–95. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F.; da Silva Cunha, I.B.; Marcucci, M.C.; Aidar, D.S.; Silva, E.C.A.; Carvalho, C.A.L.; Eberlin, M.N. Electrospray ionization mass spectrometry fingerprinting of propolis of native Brazilian stingless bees. Apidologie 2007, 38, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.P.; Huang, S.; Wei, W.T.; Ping, S.; Shen, X.G.; Li, Y.J.; Hu, F.L. Development of high-performance liquid chromatographic for quality and authenticity control of Chinese propolis. J. Food Sci. 2014, 79, C1315–C1322. [Google Scholar]
Sample Availability: Not available. |







| Compounds | LogD (pH 7.4) a |
|---|---|
| Caffeic acid | −1.74 |
| p-Coumaric acid | −1.32 |
| Isoferulic acid | −1.18 |
| Dimethoxycinnamic acid | −0.84 |
| Cinnamic acid | −0.66 |
| Kaempferol | 0.81 |
| Caffeic acid phenethyl ester | 3.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Tu, X.; Wu, D.; Gao, Z.; Wu, S.; Huang, S. Comparison of the Partition Efficiencies of Multiple Phenolic Compounds Contained in Propolis in Different Modes of Acetonitrile–Water-Based Homogenous Liquid–Liquid Extraction. Molecules 2019, 24, 442. https://doi.org/10.3390/molecules24030442
Chen W, Tu X, Wu D, Gao Z, Wu S, Huang S. Comparison of the Partition Efficiencies of Multiple Phenolic Compounds Contained in Propolis in Different Modes of Acetonitrile–Water-Based Homogenous Liquid–Liquid Extraction. Molecules. 2019; 24(3):442. https://doi.org/10.3390/molecules24030442
Chicago/Turabian StyleChen, Wenbin, Xijuan Tu, Dehui Wu, Zhaosheng Gao, Siyuan Wu, and Shaokang Huang. 2019. "Comparison of the Partition Efficiencies of Multiple Phenolic Compounds Contained in Propolis in Different Modes of Acetonitrile–Water-Based Homogenous Liquid–Liquid Extraction" Molecules 24, no. 3: 442. https://doi.org/10.3390/molecules24030442