Morphology-Dependent Catalytic Activity of Ru/CeO2 in Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. TEM and High-Resolution Transmission Electron Microscopy (HRTEM) Analysis
2.1.2. X-ray Diffraction
2.1.3. N2 Adsorption–Desorption
2.1.4. H2-Temperature Programmed Reduction
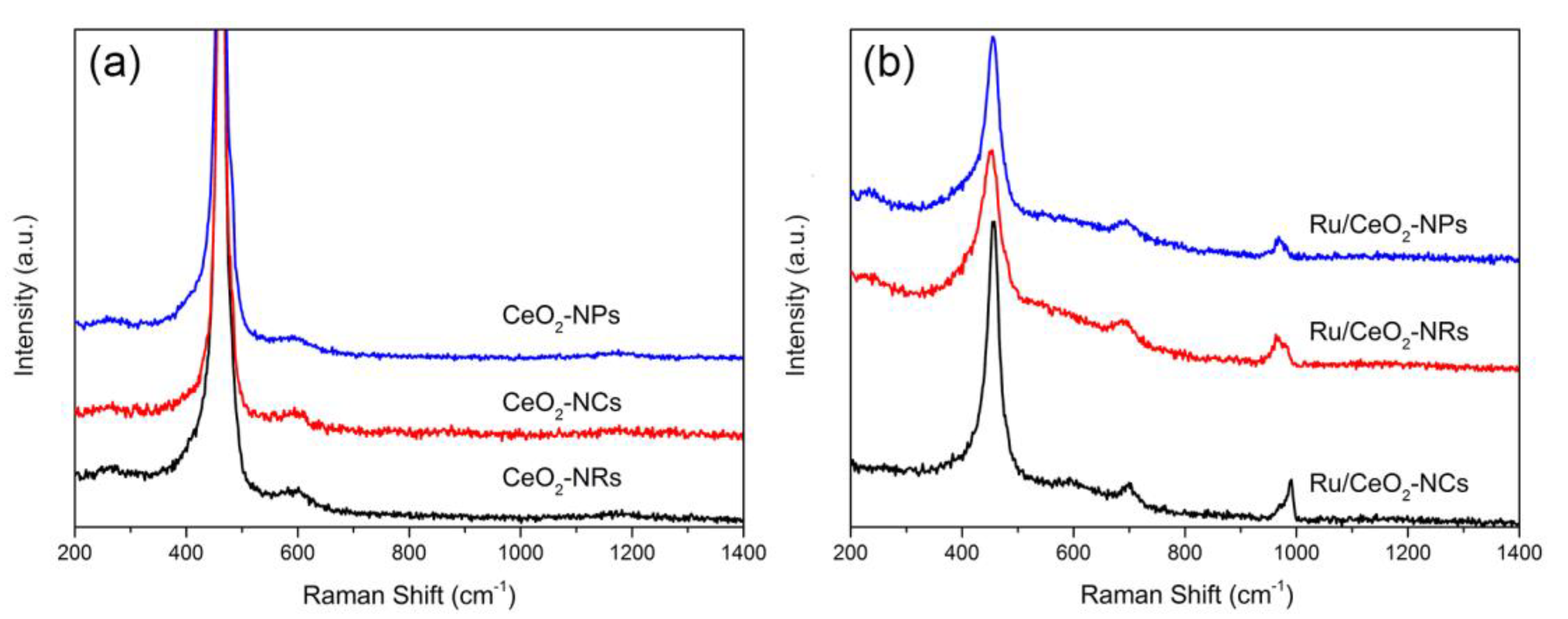
2.1.5. Raman Spectroscopy
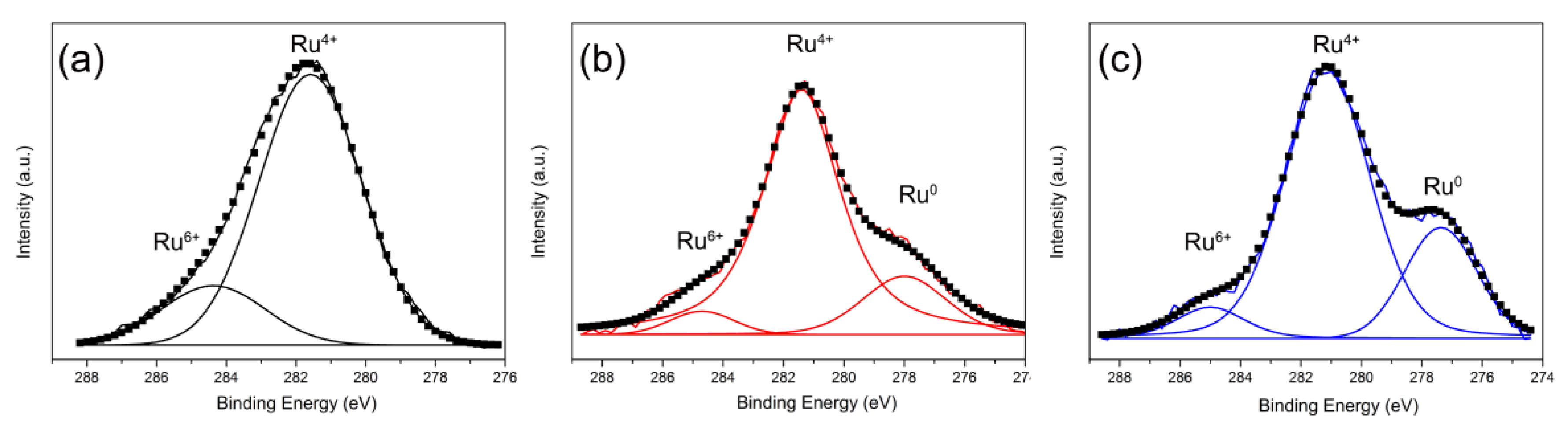
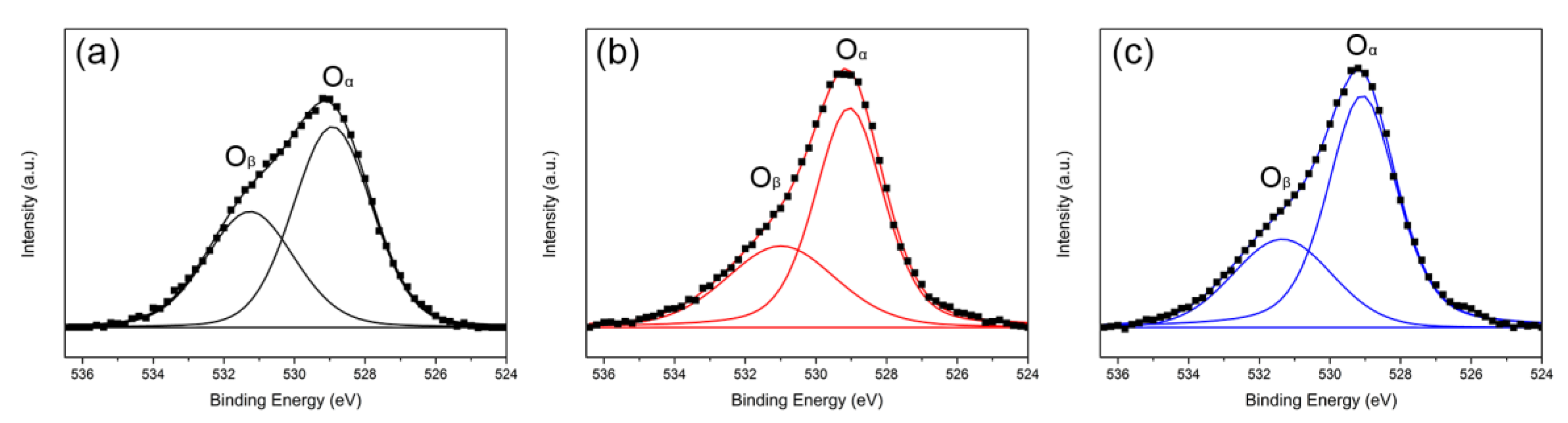
2.1.6. X-ray Photoelectron Spectroscopy (XPS)
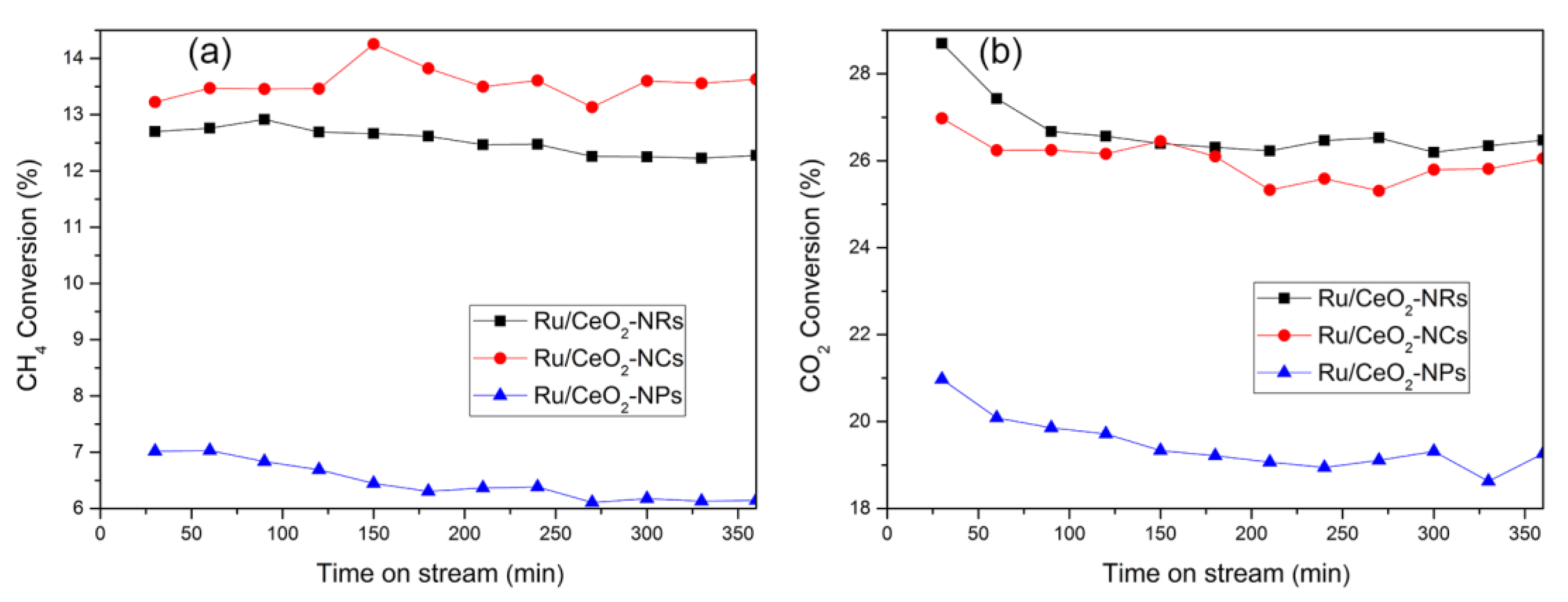
2.2. Catalytic Performance for the Dry Reforming of Methane
3. Experimental Section
3.1. Preparation of CeO2 Samples
3.2. Preparation of Ru/CeO2 Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Florides, G.A.; Christodoulides, P.; Messaritis, V. Reviewing the effect of CO2 and the sun on global climate. Renew. Sustain. Energy Rev. 2013, 26, 639–651. [Google Scholar] [CrossRef]
- Ghoniem, A.F. Needs, resources and climate change: Clean and efficient conversion technologies. Prog. Energy Combust. Sci. 2011, 37, 15–51. [Google Scholar] [CrossRef]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.; Dang, V.Q.; Lourdes, F.V.; Enas, N.; Mohammad, R.M.A.-Z. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. J. CO2 Util. 2013, 3–4, 65–73. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.; Saw, E.T. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef] [PubMed]
- Spallina, V.; Melchiori, T.; Gallucci, F.; Annaland, M.v.S. Auto-thermal reforming using mixed ion-electronic conducting ceramic membranes for a small-scale H2 production plant. Molecules 2015, 20, 4998–5023. [Google Scholar] [CrossRef]
- Abbas, H.F.; Daud, W.M.A.W. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Ma, Z.; Zhou, S. Enhanced stability of CaO and/or La2O3 promoted Pd/Al2O3 egg-shell catalysts in partial oxidation of methane to syngas. Molecules 2013, 18, 8289–8297. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Ha, K.-S.; Bae, J.W.; Woo, K.-J.; Jun, K.-W. Efficient utilization of greenhouse gas in a gas-to-liquids process combined with carbon dioxide reforming of methane. Environ. Sci. Technol. 2010, 44, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, Y.X.; Xie, Y.B.; Liu, C.J. Carbon dioxide reforming of methane over glow discharge plasma-reduced Ir/Al2O3 catalyst. Catal. Commun. 2008, 9, 1558–1562. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Sonochemical synthesis of Pt, Ru doped TiO2 for methane reforming. Appl. Catal. A 2016, 518, 102–114. [Google Scholar] [CrossRef]
- Derk, A.R.; Moore, G.M.; Sharma, S.; McFarland, E.W.; Metiu, H. Catalytic dry reforming of methane on ruthenium-doped ceria and ruthenium supported on ceria. Top. Catal. 2014, 57, 118–124. [Google Scholar] [CrossRef]
- Whang, H.S.; Choi, M.S.; Lim, J.; Kim, C.; Heo, I.; Chang, T.-S.; Lee, H. Enhanced activity and durability of Ru catalyst dispersed on zirconia for dry reforming of methane. Catal. Today 2017, 293, 122–128. [Google Scholar] [CrossRef]
- Cai, W.; Ye, L.; Zhang, L.; Ren, Y.; Yue, B.; Chen, X.; He, H. Highly dispersed nickel-containing mesoporous silica with superior stability in carbon dioxide reforming of methane: The effect of anchoring. Materials 2014, 7, 2340–2355. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO-MgO solid solution prepared by sol-gel method as precursor for Ni/MgO methane dry reforming catalyst: Effect of calcination temperature on catalytic performance. Catal. Lett. 2016, 146, 238–248. [Google Scholar] [CrossRef]
- Wei, J.M.; Xu, B.Q.; Li, J.L.; Cheng, Z.X.; Zhu, Q.M. Highly active and stable Ni/ZrO2 catalyst for syngas production by CO2 reforming of methane. Appl. Catal. A 2000, 196, L167–L172. [Google Scholar] [CrossRef]
- Carrara, C.; Munera, J.; Lombardo, E.A.; Cornaglia, L.M. Kinetic and stability studies of Ru/La2O3 used in the dry reforming of methane. Top. Catal. 2008, 51, 98–106. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K. Influence of reduction temperature on the catalytic behavior of Co/TiO2 catalysts for CH4/CO2 reforming and its relation with titania bulk crystal structure. J. Catal. 2005, 230, 75–85. [Google Scholar] [CrossRef]
- Lovell, E.C.; Scott, J.; Amal, R. Ni-SiO2 catalysts for the carbon dioxide reforming of methane: Varying support properties by flame spray pyrolysis. Molecules 2015, 20, 4594–4609. [Google Scholar] [CrossRef] [PubMed]
- Boudart, M. Catalysis by supported metals. Adv. Catal. 1969, 20, 153–166. [Google Scholar]
- Wang, H.Y.; Ruckenstein, E. Carbon dioxide reforming of methane to synthesis gas over supported rhodium catalysts: The effect of support. Appl. Catal. A 2000, 204, 143–152. [Google Scholar] [CrossRef]
- Fu, Q.; Weber, A.; Flytzani-Stephanopoulos, M. Nanostructured Au-CeO2 catalysts for low-temperature water-gas shift. Catal. Lett. 2001, 77, 87–95. [Google Scholar] [CrossRef]
- Kurnatowska, M.; Mista, W.; Mazur, P.; Kepinski, L. Nanocrystalline Ce1-xRuxO2—Microstructure, stability and activity in CO and soot oxidation. Appl. Catal. B 2014, 148, 123–135. [Google Scholar] [CrossRef]
- Devaiah, D.; Reddy, L.H.; Park, S.-E.; Reddy, B.M. Ceria-zirconia mixed oxides: Synthetic methods and applications. Catal. Rev. Sci. Eng. 2018, 60, 177–277. [Google Scholar] [CrossRef]
- Xu, S.; Yan, X.; Wang, X. Catalytic performances of NiO-CeO2 for the reforming of methane with CO2 and O2. Fuel 2006, 85, 2243–2247. [Google Scholar] [CrossRef]
- Hou, T.; Yu, B.; Zhang, S.; Xu, T.; Wang, D.; Cai, W. Hydrogen production from ethanol steam reforming over Rh/CeO2 catalyst. Catal. Commun. 2015, 58, 137–140. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, Y.; Cai, W.; Yue, B.; He, y. Promotional effect of cerium on nickel-containing mesoporous silica for carbon dioxide reforming of methane. Sci. Chin. Chem. 2015, 58, 148–155. [Google Scholar] [CrossRef]
- Liao, Y.; He, L.; Man, C.; Chen, L.; Fu, M.; Wu, J.; Ye, D.; Huang, B. Diameter-dependent catalytic activity of ceria nanorods with various aspect ratios for toluene oxidation. Chem. Eng. J. 2014, 256, 439–447. [Google Scholar] [CrossRef]
- Raju, G.; Devaiah, D.; Reddy, P.S.; Rao, K.N.; Reddy, B.M. Hydrogenolysis of bioglycerol to 1,2-propanediol over Ru/CeO2 catalysts: Influence of CeO2 characteristics on catalytic performance. Appl. Petrochem. Res. 2014, 4, 297–304. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Gao, F.; Kovarik, L.; Peden, C.H.F.; Wang, Y. Effects of CeO2 support facets on VOx/CeO2 catalysts in oxidative dehydrogenation of methanol. J. Catal. 2014, 315, 15–24. [Google Scholar] [CrossRef]
- Singhania, N.; Anumol, E.A.; Ravishankar, N.; Madras, G. Influence of CeO2 morphology on the catalytic activity of CeO2-Pt hybrids for CO oxidation. Dalton Trans. 2013, 42, 15343–15354. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meeprasert, J.; Maitarad, P.; Nammuangruk, S.; Shi, L.; Zhang, D. Investigation of the facet-dependent catalytic performance of Fe2O3/CeO2 for the selective catalytic reduction of NO with NH3. J. Phys. Chem. C 2016, 120, 1523–1533. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Gawade, P.; Mirkelamoglu, B.; Ozkan, U.S. The role of support morphology and impregnation medium on the water gas shift activity of ceria-supported copper catalysts. J. Phys. Chem. C 2010, 114, 18173–18181. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, X.; Wei, M.; Evans, D.G.; Duan, X. Catalytic behavior of supported Ru nanoparticles on the {100}, {110}, and {111} facet of CeO2. J. Catal. 2015, 329, 177–186. [Google Scholar] [CrossRef]
- Wang, Z.L.; Feng, X.D. Polyhedral shapes of CeO2 nanoparticles. J. Phys. Chem. B 2003, 107, 13563–13566. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Ren, M.; Sun, G.; Gao, D.; Chong, Y.R.C.; Li, X.; Chen, G. Effect of ceria morphology on the catalytic activity of Ru/ceria for the dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2017, 42, 6757–6764. [Google Scholar] [CrossRef]
- Wang, Z.L. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Oxidising CO to CO2 using ceria nanoparticles. Phys. Chem. Chem. Phys. 2005, 7, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, Z.; Deng, Y.; Gao, F.; Liu, B.; Dong, L. Morphology and crystal-plane effects of nanoscale ceria on the activity of CuO/CeO2 for NO reduction by CO. ChemCatChem 2011, 3, 978–989. [Google Scholar] [CrossRef]
- Schierbaum, K.D. Ordered ultra-thin cerium oxide overlayers on Pt(111) single crystal surfaces studied by LEED and XPS. Surf. Sci. 1998, 399, 29–38. [Google Scholar] [CrossRef]
- YAO, H.C.; YAO, Y.F.Y. Ceria in automotive exhaust catalysts. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Weber, W.H.; Bass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 179–184. [Google Scholar] [CrossRef]
- Ji, S.; Xiao, Z.; Zhang, H.; Li, L.; Li, G.; Wang, L.; Liu, G. Catalytic steam reforming of n-dodecane over high surface area Ce0.75Zr0.25O2 supported Ru catalysts. Int. J. Hydrogen Energy 2017, 42, 29484–29497. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Sample | Specific Surface Area a (m2·g−1) | Particle Size b (nm) | Mean Sizes c (nm) | Ru d (wt.%) | Ru Dispersion e (%) | |
|---|---|---|---|---|---|---|
| CeO2-NRs | 87.1 | (8 ± 2) × (50 − 100) | 13.6 | - | ||
| CeO2-NCs | 31.7 | 26 ± 15 | 23.0 | - | ||
| CeO2-NPs | 67.4 | 13 ± 5 | 13.9 | - | ||
| Ru/CeO2-NRs | 84.9 | (7 ± 3) × (20 − 100) | 12.1 | 2.4 | 54 | |
| Ru/CeO2-NCs | 30.1 | 24 ± 15 | 23.6 | 2.4 | 59 | |
| Ru/CeO2-NPs | 65.8 | 12 ± 5 | 13.7 | 2.3 | 35 | |
| Sample | I(594 + 1170)/I461 | I(694 + 968)/I461 |
|---|---|---|
| CeO2-NRs | 0.116 | - |
| CeO2-NCs | 0.102 | - |
| CeO2-NPs | 0.057 | - |
| Ru/CeO2-NRs | - | 0.234 |
| Ru/CeO2-NCs | - | 0.187 |
| Ru/CeO2-NPs | - | 0.177 |
| Samples | Ru4+ (%) | Ce3+/(Ce3+ + Ce4+) (%) | Oβ/Oα |
|---|---|---|---|
| Ru/CeO2-NRs | 80.9 | 22.8 | 0.40 |
| Ru/CeO2-NCs | 78.9 | 21.1 | 0.36 |
| Ru/CeO2-NPs | 22.4 | 19.8 | 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Ren, Y.; Fu, Y.; Yue, B.; Tsang, S.C.E.; He, H. Morphology-Dependent Catalytic Activity of Ru/CeO2 in Dry Reforming of Methane. Molecules 2019, 24, 526. https://doi.org/10.3390/molecules24030526
He L, Ren Y, Fu Y, Yue B, Tsang SCE, He H. Morphology-Dependent Catalytic Activity of Ru/CeO2 in Dry Reforming of Methane. Molecules. 2019; 24(3):526. https://doi.org/10.3390/molecules24030526
Chicago/Turabian StyleHe, Lulu, Yuanhang Ren, Yingyi Fu, Bin Yue, Shik Chi Edman Tsang, and Heyong He. 2019. "Morphology-Dependent Catalytic Activity of Ru/CeO2 in Dry Reforming of Methane" Molecules 24, no. 3: 526. https://doi.org/10.3390/molecules24030526





