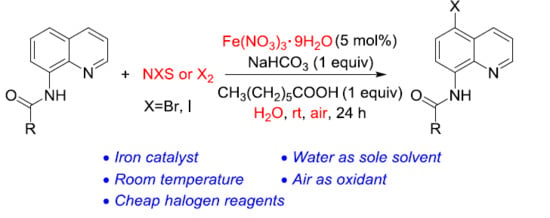Iron(III)-Catalyzed Highly Regioselective Halogenation of 8-Amidoquinolines in Water
Abstract
:1. Introduction
2. Results and Discussion

3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis of Starting Materials
3.3. General Procedures for Iron-Catalyzed Halogenation C5-H of 8-Amidoquinolines under Mild Conditions in Water
3.4. General Procedures for Suzuki Coupling Reaction of N-(5-Bromoquinolin-8-yl)pivalamide (4a as an Example)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snieckus, V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 1990, 90, 879–933. [Google Scholar] [CrossRef]
- Shiraki, H.; Kozar, M.P.; Melendez, V.; Hudson, T.H.; Ohrt, C.; Magill, A.J.; Lin, A.J. Antimalarial Activity of Novel 5-Aryl-8-Aminoquinoline Derivatives. J. Med. Chem. 2011, 54, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Vangapandu, S.; Sachdeva, S.; Jain, R. Synthesis and blood-schizontocidal antimalarial activities of 2-substituted/2,5-disubstituted-8-quinolinamines and some of their amino acid conjugates. Bioorganic Med. Chem. 2004, 12, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.J.; Ross, D.C.; Adams, P.A. Quinoline anti-malarial drugs inhibit spontaneous formation of β-haematin (malaria pigment). FEBS Lett. 1994, 352, 54–57. [Google Scholar] [CrossRef]
- Foley, M.; Tilley, L. Quinoline Antimalarials: Mechanisms of Action and Resistance and Prospects for New Agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar] [CrossRef]
- Hughes, G.; Bryce, M.R. Electron-transporting materials for organic electroluminescent and electrophosphorescent devices. J. Mater. Chem. 2005, 15, 94–107. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Quinoline as a Privileged Scaffold in Cancer Drug Discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Zaitsev, V.G.; Shabashov, D.; Daugulis, O. Highly Regioselective Arylation of sp3 C-H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. [Google Scholar] [CrossRef]
- Rouquet, G.; Chatani, N. Catalytic Founctionalization of C(sp2)-H and C(sp3)-H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef]
- Zhu, C.; Yi, M.; Wei, D.; Chen, X.; Wu, Y.; Cui, X. Copper-Catalyzed Direct Amination of Quinoline N-Oxides via C–H Bond Activation under Mild Conditions. Org. Lett. 2014, 16, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, C.; Cui, X.; Wu, Y. Direct 2-acetoxylation of quinoline N-oxides via copper catalyzed C–H bond activation. Chem. Commun. 2013, 49, 6900–6902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, X.; Pei, Y.; Zhang, Q.; Bai, J.; Wei, D.; Wu, Y. Direct regioselective phosphonation of heteroaryl N-oxides with H-phosphonates under metal and external oxidant free conditions. Chem. Commun. 2014, 50, 14409–14411. [Google Scholar] [CrossRef]
- Li, G.; Jia, C.; Sun, K. Copper-Catalyzed Intermolecular Dehydrogenative Amidation/Amination of Quinoline N-Oxides with Lactams/Cyclamines. Org. Lett. 2013, 15, 5198–5201. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.C.; Stuart, D.R.; Leclerc, J.P.; Bertrand-Laperle, M.; Villemure, E.; Sun, H.Y.; Lasserre, S.; Guimond, N.; Lecavallier, M.; Fagnou, K. Palladium-Catalyzed Direct Arylation of Azine and Azole N-Oxides: Reaction Development, Scope and Applications in Synthesis. J. Am. Chem. Soc. 2009, 131, 3291–3306. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Lv, Y.; Wang, J.; Sun, J.; Liu, L.; Jia, M.; Liu, X.; Li, Z.; Wang, X. Regioselective, Molecular Iodine-Mediated C3 Iodination of Quinolines. Org. Lett. 2015, 17, 4408–4411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Qi, Z.S.; Li, X.W. Rhodium(III)-Catalyzed C–C and C–O Coupling of Quinoline N-Oxides with Alkynes: Combination of C–H Activation with O-Atom Transfer. Angew. Chem. Int. Ed. 2014, 53, 10794–10798. [Google Scholar] [CrossRef]
- Jeong, J.; Patel, P.; Hwang, H.; Chang, S. Rhodium(III)-Catalyzed C–C Bond Formation of Quinoline N-Oxides at the C-8 Position under Mild Conditions. Org. Lett. 2014, 16, 4598–4601. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, J.; Jeong, J.; Chang, S. Regioselective Introduction of Heteroatoms at the C-8 Position of Quinoline N-Oxides: Remote C–H Activation Using N-Oxide as a Stepping Stone. J. Am. Chem. Soc. 2014, 136, 10770–10776. [Google Scholar] [CrossRef]
- Suess, A.M.; Ertem, M.Z.; Cramer, C.J.; Stahl, S.S. Divergence between Organometallic and Single-Electron-Transfer Mechanisms in Copper(II)-Mediated Aerobic C–H Oxidation. J. Am. Chem. Soc. 2013, 135, 9797–9804. [Google Scholar] [CrossRef]
- Guo, H.; Chen, M.; Jiang, P.; Chen, J.; Pan, L.; Wang, M.; Xie, C.; Zhang, Y. Copper and palladium mediated C–H chlorination on 8-acylaminoquinoline scaffolds. Tetrahedron 2015, 71, 70–76. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Zhou, G.; Ying, B.; Ye, P.; Su, L.; Shen, C.; Zhang, P. Copper(II)-catalyzed C5 and C7 halogenation of quinolines using sodium halides under mild conditions. Org. Biomol. Chem. 2016, 14, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, W.; Ye, K.; Li, J. The Highly Regioselective Halogenation of N-(8-quinolinyl)amides on the C-5 Position with Cuprous Halides Under Mild Conditions. ChemistrySelect 2016, 1, 5874–5878. [Google Scholar] [CrossRef]
- Liu, X.X.; Wu, Z.Y.; Luo, X.L.; He, Y.Q.; Zhou, X.Q.; Fan, Y.X.; Huang, G.S. PhI(OAc)2 oxidative C5 halogenation of quinolines using copper halides under mild conditions. RSC Adv. 2016, 6, 71485–71488. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, H.; Wu, Q.; He, M.; Li, P.; Su, Q.; Mu, Y. Copper-Catalyzed Regioselective C–H Iodination of Aromatic Carboxamides. Synlett 2016, 27, 868–875. [Google Scholar] [CrossRef]
- He, X.; Xu, Y.Z.; Kong, L.X.; Wu, H.H.; Ji, D.Z.; Wang, Z.B.; Xu, Y.G.; Zhu, Q.H. Copper(I) and N-fluorobenzenesulfonimide-mediated direct regioselective halogenation of 8-amidoquinolines on the C5 position. Org. Chem. Front. 2017, 4, 1046–1050. [Google Scholar] [CrossRef]
- Rao, N.S.; Reddy, G.M.; Sridhar, B.; Sarma, M.H. Copper-Mediated Remote Highly Site-Selective C–H Bond Bromination and Chlorination of Quinolines at the C5 Position that is Geometrically Difficult to Access. Eur. J. Org. Chem. 2017, 438–442. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Jiang, K.; Zhang, Q.; Li, D. Transition-metal-free oxidative C5 C–H-halogenation of 8-aminoquinoline amides using sodium halides. Org. Biomol. Chem. 2016, 14, 10180–10184. [Google Scholar] [CrossRef]
- Jiao, J.Y.; Mao, Y.J.; Feng, A.W.; Li, X.F.; Li, M.T.; Zhang, X.H. The regioselective C5 halogenation of quinolines using sodium halides under transition metal-free conditions. Tetrahedron 2017, 73, 1482–1488. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Cao, X.; Au, C.T.; Qiu, R.; Yin, S.F. Metal–free C5-H Bromination of Quinolines for One-pot C-X (X=C, O, S) Bond Formations. Adv. Synth. Catal. 2017, 359, 2864–2873. [Google Scholar] [CrossRef]
- Motati, D.R.; Uredi, D.; Watkins, E.B. A general method for the metal-free, regioselective, remote C-H halogenation of 8-substituted quinolines. Chem. Sci. 2018, 9, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, T.; Liu, Y.; Wang, T.; Lin, A.; Yao, H.; Xu, J. Metal-free C5-selective halogenation of quinolines under aqueous conditions. Org. Chem. Front. 2017, 4, 622–626. [Google Scholar] [CrossRef]
- Liang, L.; Li, Z.; Zhou, X. Pyridine N-Oxides as Ligands in Cu-Catalyzed N-Arylation of Imidazoles in Water. Org. Lett. 2009, 11, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. An Efficient Copper-Catalyzed Carbon−Sulfur Bond Formation Protocol in Water. Org. Lett. 2011, 13, 454–457. [Google Scholar] [CrossRef]
- Li, Z.; Ke, F.; Deng, H.; Xu, H.; Xiang, H.; Zhou, X. Synthesis of disulfides and diselenides by copper-catalyzed coupling reactions in water. Org. Biomol. Chem. 2013, 11, 2943–2946. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luo, F.; Chen, S.; Li, Z.; Xiang, H.; Zhou, X. Palladium-catalyzed para-selective arylation of phenols with aryl iodides in water. Chem. Commun. 2013, 49, 7653–7655. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, L.; Wu, Z.; Li, Z.; Xiang, H.; Zhou, X. Quaternary ammonium salt as alkylation agent in three-component reactions for the synthesis of benzothiazoles in water. RSC Adv. 2014, 4, 27775–27779. [Google Scholar] [CrossRef]
- Qu, Y.; Ke, F.; Zhou, L.; Li, Z.; Xiang, H.; Wu, D.; Zhou, X. Synthesis of 3-indole derivatives by copper sulfonato Salen catalyzed three-component reactions in water. Chem. Commun. 2011, 47, 3912–3914. [Google Scholar] [CrossRef]
- Jing, L.; Wei, J.; Zhou, L.; Huang, Y.; Li, Z.; Zhou, X. Lithium pipecolinate as a facile and efficient ligand for copper-catalyzed hydroxylation of aryl halides in water. Chem. Commun. 2010, 46, 4767–4769. [Google Scholar] [CrossRef]
- Yao, X.; Weng, X.; Wang, K.; Xiang, H.; Zhou, X. Transition metal free oxygenation of 8-aminoquinoline amides in water. Green Chem. 2018, 20, 2472–2476. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, S.; Yang, F.; Zhu, Y.; Kang, J.; Wu, Y.; Wu, Y. Merging Photoredox Catalysis with Iron(III) Catalysis: C5-H Bromination and Iodination of 8-Aminoquinoline Amides in Water. Adv. Synth. Catal. 2017, 359, 1976–1980. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent development and application of chiral phase-transfer catalysts. Chem. Rev. 2007, 107, 5656–5682. [Google Scholar] [CrossRef] [PubMed]
- Merkushev, E.B. Advances in the Synthesis of Iodoaromatic Compounds. Synthesis 1988, 12, 923–937. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Mathew, T.; Hoole, D.; Esteves, P.M.; Wang, Q.; Rasul, G.; Olah, G.A. N-Halosuccinimide/BF3-H2O, Efficient Electrophilic Halogenating Systems for Aromatics. J. Am. Chem. Soc. 2004, 126, 15770–15776. [Google Scholar] [CrossRef] [PubMed]
- Zawartka, W.; Póspiech, P.; Cypryk, M.; Trzeciak, A.M. Palladium supported on aminopropyl-functionalized polymethylsiloxane microspheres: Simple and effective catalyst for the Suzuki–Miyaura C–C coupling. J. Mol. Catal. A Chem. 2015, 407, 230–235. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. An efficient protocol for palladium-catalyzed ligand-free Suzuki–Miyaura coupling in water. Green Chem. 2012, 14, 1873–1876. [Google Scholar] [CrossRef]
- Cui, M.; Liu, J.; Lu, X.; Lu, X.; Zhang, Z.; Xiao, B.; Fu, Y. Iron-mediated remote C-H bond benzylation of 8-aminoquinoline amides. Tetrahedron Lett. 2017, 58, 1912–1916. [Google Scholar] [CrossRef]
- Liang, H.; Jiang, K.; Ding, W.; Yuan, Y.; Shuai, L.; Chen, Y.; Wei, Y. Selective remote C-H sulfonylation of aminoquinolines with arylsulfonyl chlorides via copper catalysis. Chem. Commun. 2015, 51, 16928–16931. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |











| Entry | Catalyst (%) | Additive | Yield [%] b | |
|---|---|---|---|---|
| Using Br2 | Using NBS | |||
| 1 c | - | - | 0 | 0 |
| 2 | - | - | 35 | 91 |
| 3 | Pd(OAc)2 (10) | - | 47 | 67 |
| 4 | Cu(OAc)2 (20) | - | 43 | 65 |
| 5 | Co(OAc)2·9H2O (20) | - | 39 | 58 |
| 6 | Fe(NO3)3·9H2O (5) | - | 68 | 68 |
| 7 | FeCl3·9H2O (5) | - | 63 | 65 |
| 8 | Fe(NO3)3·9H2O (5) | Ag2O | 59 | 65 |
| 9 | Fe(NO3)3·9H2O (5) | Ag2CO3 | 64 | 69 |
| 10 | Fe(NO3)3·9H2O (5) | AgOAc | 73 | 75 |
| 11 | Fe(NO3)3·9H2O (5) | CH3(CH2)5COOAg | 89 | 90 |
| 12 | Fe(NO3)3·9H2O (5) | CH3(CH2)5COOH, NaHCO3 d | 95 | 95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Pan, L.; Zhou, X. Iron(III)-Catalyzed Highly Regioselective Halogenation of 8-Amidoquinolines in Water. Molecules 2019, 24, 535. https://doi.org/10.3390/molecules24030535
Long Y, Pan L, Zhou X. Iron(III)-Catalyzed Highly Regioselective Halogenation of 8-Amidoquinolines in Water. Molecules. 2019; 24(3):535. https://doi.org/10.3390/molecules24030535
Chicago/Turabian StyleLong, Yang, Lei Pan, and Xiangge Zhou. 2019. "Iron(III)-Catalyzed Highly Regioselective Halogenation of 8-Amidoquinolines in Water" Molecules 24, no. 3: 535. https://doi.org/10.3390/molecules24030535