Profiling Volatile Constituents of Homemade Preserved Foods Prepared in Early 1950s South Dakota (USA) Using Solid-Phase Microextraction (SPME) with Gas Chromatography–Mass Spectrometry (GC-MS) Determination
Abstract
:1. Introduction
2. Results and Discussion
2.1. SPME Method Development
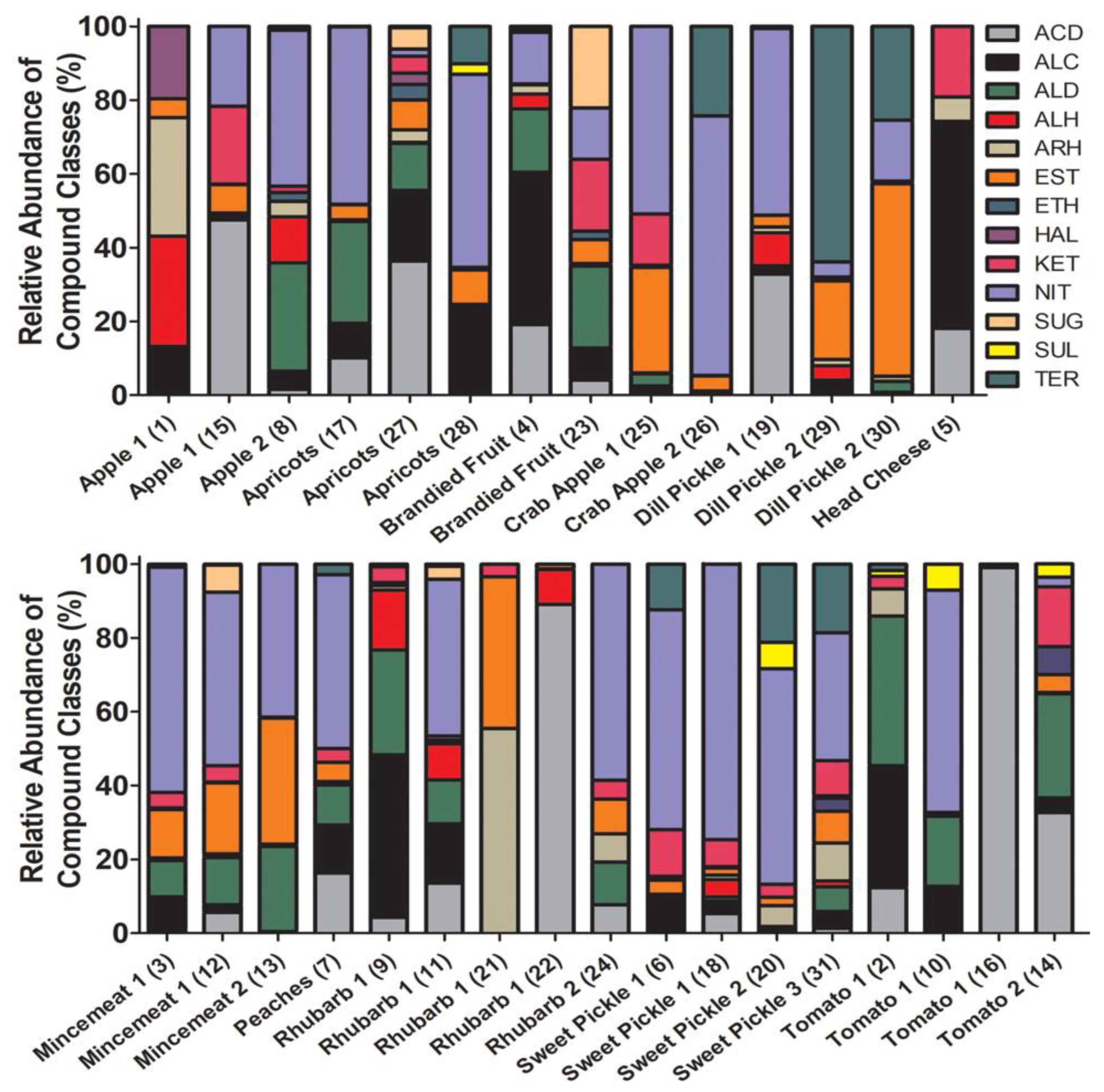
2.2. SPME Analysis of 1950s Preserves
2.3. BPA in 1950s Preserves
3. Experimental Section
3.1. Chemicals, Equipment, and Ingredients
3.2. Sample Collection, Curation, and Preparation
3.3. SPME Analysis
3.4. SPME QA/QC
3.5. GC-MS Analysis
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawless, H.T. Blackwell Handbook of Sensation and Perception; Goldstein, E.B., Ed.; Blackwell Publishing: Malden, MA, USA, 2005; pp. 601–635. [Google Scholar]
- Ma, X.; Su, M.; Wu, H.; Zhou, Y.; Wang, S. Analysis of the Volatile Profile of Core Chinese Mango Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Molecules 2018, 23, 1480. [Google Scholar] [CrossRef]
- Costa, F.; Cappellin, L.; Zini, E.; Patocchi, A.; Kellerhals, M.; Komjanc, M.; Gessler, C.; Biasioli, F. QTL Validation and Stability for Volatile Organic Compounds (VOCs) in Apple. Plant Sci. 2013, 211, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.J. Aroma Chemicals for Savory Flavors. Perfume. Flavor. 1998, 23, 9–18. [Google Scholar]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, H. Drying Technologies of Foods—Their History and Future. Dry. Technol. 1989, 7, 315–369. [Google Scholar] [CrossRef]
- Prajapati, J.B.; Nair, B.M. The History of Fermented Foods. In Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–22. [Google Scholar]
- Graham, J.C. The French Connection in the Early History of Canning. J. Royal Soc. Med. 1981, 74, 374–381. [Google Scholar] [Green Version]
- Kreidberg, M. Food on the Frontier; Minnesota Historical Society: Saint Paul, MN, USA, 1975; p. 324. [Google Scholar]
- Evershed, R.P.; Heron, C.; Charters, S.; Goad, L.G. The Survival of Food Residues: New Methods of Analysis, Interpretation and Application. Proc. British Acad. 1991, 77, 187–208. [Google Scholar]
- Evershed, R.P.; Bland, H.A.; van Bergen, P.F.; Carter, J.F.; Horton, M.C.; Rowley-Conwy, P.A. Volatile Compounds in Archaeological Plant Remains and the Maillard Reaction During Decay of Organic Matter. Science 1997, 278, 432–433. [Google Scholar] [CrossRef]
- Perrault, K.A.; Stefanuto, P.; Dubois, L.; Cnuts, D.; Rots, V.; Focant, J. A New Approach for the Characterization of Organic Residues from Stone Tools Using GCGC-TOFMS. Separations 2016, 3, 16. [Google Scholar] [CrossRef]
- Regert, M.; Alexandre, V.; Thomas, N.; Lattuati-Derieux, A. Molecular Characterisation of Birch Bark Tar by Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry: A New Way for Identifying Archaeological Glues. J. Chromatogr. 2006, 1101, 245–253. [Google Scholar] [CrossRef]
- Cnuts, D.; Perrault, K.A.; Stefanuto, P.-H.; Dubois, L.M.; Focant, J.-F.; Rots, V. Fingerprinting Glues Using HS-SPME GCxGC-HRTOFMS: A New Powerful Method Allows Tracking Glues Back in Time. Archaeometry 2018, 60, 1361–1376. [Google Scholar] [CrossRef]
- Evershed, R.P. Organic Residue Analysis in Archaeology: The Archaeology Biomarker Revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- Evershed, R.P.; Heron, C.; Goad, L.G. Epicuticular Wax Components Preserved in Potsherds as Chemical Indicators of Leafy Vegetables in Ancient Diets. Antiquity 1991, 65, 540–544. [Google Scholar] [CrossRef]
- Evershed, R.P.; Heron, C.; Goad, L.J. Analysis of Organic Residues of Archaeological Origin by High Temperature Gas Chromatography/Mass Spectrometry. Analyst 1990, 115, 1339–1342. [Google Scholar] [CrossRef]
- Craig, O.E.; Chapman, J.; Heron, C.; Willis, L.H.; Bartosiewicz, L.; Taylor, G.; Whittle, A.; Collins, M. Did the First Farmers of Central and Eastern Europe Produce Dairy Foods? Antiquity 2005, 79, 882–894. [Google Scholar] [CrossRef]
- Oudemans, T.F.M.; Boon, J.J. Molecular Archaeology: Analysis of Charred Food Remains from Prehistoric Pottery by Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Anal. Appl. Pyrol. 1991, 20, 197–227. [Google Scholar] [CrossRef]
- Barnard, H.; Ambrose, S.H.; Beehr, D.E.; Forster, M.D.; Lanehart, R.E.; Malainey, M.E.; Parr, R.E.; Rider, M.; Solazzo, C.; Yohe, R.M. Mixed Results of Seven Methods for Organic Residue Analysis Applied to One Vessel with the Residue of a Known Foodstuff. J. Archaeol. Sci. 2007, 34, 28–37. [Google Scholar] [CrossRef]
- Palma-Harris, C.; McFeeters, R.F.; Fleming, H.P. Solid-Phase Microextraction (SPME) Technique for Measurement of Generation of Fresh Cucumber Flavor Compounds. J. Agricult. Food Chem. 2001, 49, 4203–4207. [Google Scholar] [CrossRef]
- Legua, P.; Domenech, A.; Martinez, J.J.; Sanchez-Rodriguez, L.; Hernandez, F.; Carbonell-Barrachina, A.A.; Melgarejo, P. Bioactive and Volatile Compounds in Sweet Cherry Cultivars. J. Food Nutr. Res. 2017, 5, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Levaj, B.; Dragovic-Uzelac, V.; Delonga, K.; Ganic, K.K.; Banovic, M.; Kovacevic, D.B. Polyphenols and Volatiles in Fruits of Two Sour Cherry Cultivars, Some Berry Fruits and Their Jams. Food Technol. Biotechnol. 2010, 48, 538–547. [Google Scholar]
- Marsili, R.T.; Miller, N. Determination of Major Aroma Impact Compounds in Fermented Cucumbers by Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry–Olfactometry Detection. J. Chromatogr. Sci. 2000, 38, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Guler, Z.; Karaca, F.; Yetisir, H. Volatile Compounds in the Peel and Flesh of Cucumber (Cucumis sativus L.) Grafted onto Bottle Gourd (Lagenaria siceraria) Rootstock. J. Horticult. Sci. Biotechnol. 2013, 88, 123–128. [Google Scholar] [CrossRef]
- Maleki, G.; Sedaghat, N.; Woltering, E.J.; Farhoodi, M.; Mohebbi, M. Chitosan-Limonene Coating in Combination with Modified Atmosphere Packaging Preserve Postharvest Quality of Cucumber During Storage. J. Food Meas. Character. 2018, 12, 1610–1621. [Google Scholar] [CrossRef]
- Bruni, R.; Bianchi, A.; Bellardi, M.G. Essential Oil Composition of Agastache anethiodora Britton (Lamiaceae) Infected by Cucumber Mosaic Virus (CMV). Flav. Fragr. J. 2007, 22, 66–70. [Google Scholar] [CrossRef]
- Iranmanesh, M.; Ezzatpanah, H.; Akbari-Adergani, B.; Torshizi, M.A.K. SPME/GC-MS Characterization of Volatile Compounds of Iranian Traditional Dried Kashk. Int. J. Food Prop. 2018, 21, 1067–1079. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A.; Teranishi, R.; Buttery, R.G. Meat Flavor Volatiles: A Review of the Composition, Techniques of Analysis, and Sensory Evaluation. CRC Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile Compounds in Honey: A Review on Their Involvement in Aroma, Botanical Origin Determination and Potential Biomedical Activities. Int. J. Molec. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulieu, J.C.; Lea, J.M. Characterization and Semiquantitative Analysis of Volatiles in Seedless Watermelon Varieties Using Solid-Phase Microextraction. J. Agric. Food Chem. 2006, 54, 7789–7793. [Google Scholar] [CrossRef]
- Malorni, L.; Martignetti, A.; Cozzolino, R. Volatile Compound Profiles by HS GC-MS for the Evaluation of Postharvest Conditions of a Peach Cultivar. Ann. Chromatogr. Sep. Tech. 2015, 1, 1007. [Google Scholar]
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Camara, J.S. Characterization of Volatile Substances in Apples from Rosaceae Family by Headspace Solid-Phase Microextraction Followed by GC-qMS. J. Sep. Sci. 2009, 32, 1875–1888. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma Characterization Based on Aromatic Series Analysis in Table Grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.J.; Ho, C. Volatile Sulfur Compounds in Tropical Fruits. J. Food Drug Anal. 2018, 26, 445–468. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.A. The Politics of Plastics: The Making and Unmaking of Bisphenol A “Safety”. Am. J. Pub. Health 2009, 99, S559–S566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Plastics and Health Risks. Ann. Rev. Pub. Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.E.; Kendig, E.L.; Belcher, S.M. Assessment of Bisphenol A Released from Reusable Plastic, Aluminium and Stainless Steel Water Bottles. Chemosphere 2011, 85, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.A.; Pawliszyn, J. Air Sampling and Analysis of Volatile Organic Compounds with Solid Phase Microextraction. J. Air Waste Manag. Assoc. 2001, 51, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Tuduri, L.; Desauziers, V.; Fanlo, J.L. Potential of Solid-Phase Microextraction Fibers for the Analysis of Volatile Organic Compounds in Air. J. Chromatogr. Sci. 2001, 39, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Augusto, F.; Valente, A.L.P. Applications of Solid-Phase Microextraction to Chemical Analysis of Live Biological Samples. Trends Anal. Chem. 2002, 21, 428–438. [Google Scholar] [CrossRef]
- Wardencki, W.; Michulec, M.; Curylo, J. A Review of Theoretical and Practical Aspects of Sold-Phase Microextraction in Food Analysis. Int. J. Food Sci. Technol. 2004, 39, 703–717. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioproc. Technol. 2011, 4, 1–26. [Google Scholar] [CrossRef]
Sample Availability: Samples of the historical preserves assessed in this study are not available from the authors. |



| Sample a | Sample Number | N b | pH | Sample Description c | Taste d |
|---|---|---|---|---|---|
| Apple 1 | 1, 15 | 2 | 3.1–3.4 | Sliced and shredded apple in opaque liquid with apple sauce consistency | Sweet |
| Apple 2 | 8 | 1 | 3.0 | Sliced apples stacked in clear liquor | Sweet |
| Apricots | 17, 27, 28 | 3 | 2.9–3.5 | Spherical, fleshy fruit bodies in dark liquid | Sweet |
| Brandied Fruit | 4, 23 | 2 | 2.9–3.3 | Dark gelatinous fruit bodies resembling plums in dark, viscous liquor | Sweet/alcohol |
| Crab Apple 1 | 25 | 1 | 3.4 | Round fleshy fruit bodies with a single stem and many small seeds in the center in dark liquid | Sweet |
| Crab Apple 2 | 26 | 1 | 3.8 | Round fleshy fruit bodies with no stem and many small seeds in the center in dark liquid | Sweet |
| Dill Pickle 1 | 19 | 1 | 3.8 | Whole cucumbers with dill plants and seeds in clear liquid—smaller jar | Salty |
| Dill Pickle 2 | 29, 30 | 2 | 3.2–3.3 | Cucumbers quartered longitudinally with dill plants and seeds in clear liquid—larger jar | Salty |
| Head Cheese | 5 | 1 | 3.7 | Cylindrical mass of spongy material resembling cheese in amber liquor | Salty/savory |
| Mincemeat 1 | 3, 12 | 2 | 3.9–4.0 | Fleshy fruit bodies resembling currants, raisins, leafage, and fleshy chunks resembling meat in small volume of clear liquid—larger jar | Sweet/savory |
| Mincemeat 2 | 13 | 1 | 4.0 | Fleshy fruit bodies resembling currants, raisins, leafage, and fleshy chunks resembling meat in small volume of clear liquid—smaller jar | Sweet/savory |
| Peaches | 7 | 1 | 3.6 | Dark, spherical, fleshy fruit bodies with a single solid pit in the center in dark liquid | Sweet |
| Rhubarb 1 | 9, 11, 21, 22 | 4 | 2.9–3.0 | Shredded vegetative material in dark liquid | Slightly sweet |
| Rhubarb 2 | 24 | 1 | 3.1 | Cylindrical mass of vegetative material in dark liquid | Slightly sweet |
| Sweet Pickle 1 | 6, 18 | 2 | 2.5–3.2 | Halved cucumbers in dark liquid | Sweet |
| Sweet Pickle 2 | 20 | 1 | 3.2 | Cucumber slices in dark liquid—smaller jar | Sweet |
| Sweet Pickle 3 | 31 | 1 | 3.2 | Cucumber slices in dark liquid—larger jar | Sweet |
| Tomato 1 | 2, 10, 16 | 3 | 4.1–4.2 | Whole and shredded tomato in opaque, red liquor with tomato soup consistency | Sweet/salty |
| Tomato 2 | 14 | 1 | 4.1 | Whole and shredded tomato in red liquor with spaghetti sauce consistency | Sweet/salty |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leinen, L.J.; Swenson, V.A.; Juntunen, H.L.; McKay, S.E.; O’Hanlon, S.M.; Videau, P.; Gaylor, M.O. Profiling Volatile Constituents of Homemade Preserved Foods Prepared in Early 1950s South Dakota (USA) Using Solid-Phase Microextraction (SPME) with Gas Chromatography–Mass Spectrometry (GC-MS) Determination. Molecules 2019, 24, 660. https://doi.org/10.3390/molecules24040660
Leinen LJ, Swenson VA, Juntunen HL, McKay SE, O’Hanlon SM, Videau P, Gaylor MO. Profiling Volatile Constituents of Homemade Preserved Foods Prepared in Early 1950s South Dakota (USA) Using Solid-Phase Microextraction (SPME) with Gas Chromatography–Mass Spectrometry (GC-MS) Determination. Molecules. 2019; 24(4):660. https://doi.org/10.3390/molecules24040660
Chicago/Turabian StyleLeinen, Lucas J., Vaille A. Swenson, Hope L. Juntunen, Scott E. McKay, Samantha M. O’Hanlon, Patrick Videau, and Michael O. Gaylor. 2019. "Profiling Volatile Constituents of Homemade Preserved Foods Prepared in Early 1950s South Dakota (USA) Using Solid-Phase Microextraction (SPME) with Gas Chromatography–Mass Spectrometry (GC-MS) Determination" Molecules 24, no. 4: 660. https://doi.org/10.3390/molecules24040660