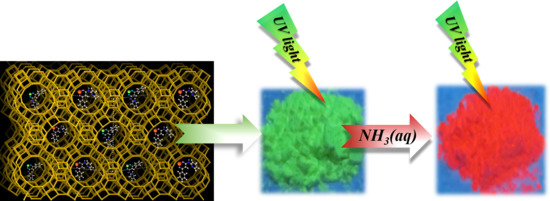
Ammonia-Responsive Luminescence of Ln3+-β-diketonate Complex Encapsulated within Zeolite Y
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of the β-diketone Ligand HPBA
3.3. Preparation of the Ln3+-Exchanged ZY
3.4. Preparation of the Luminescent Hybrid Composite Ln3+(HPBAn)@ZY
3.5. Exposure to Aqueous Ammonia (Concentration from 10−12 to 0.25 wt%)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, W.; Yu, T.; Wu, L.; Bi, L. Fabrication of white luminescence composite films containing Dy-polyoxometalate and the study of their luminescence switching behaviors. Chem. Commun. 2016, 52, 10403–10406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, Y.; Song, B.; Zhang, H.-L.; Chen, H.; Cai, H.; Liu, W.; Tang, Y. A Stimuli-Responsive Smart Lanthanide Nanocomposite for Multidimensional Optical Recording and Encryption. Angew. Chem. Int. Ed. 2017, 56, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Cho, I.; Gierschner, J.; Kim, J.H.; Kim, J.H.; Kwon, J.E.; Kwon, O.K.; Whang, D.R.; Park, J.-H.; An, B.-K.; Park, S.Y. Stimuli-Responsive Reversible Fluorescence Switching in a Crystalline Donor-Acceptor Mixture Film: Mixed Stack Charge-Transfer Emission versus Segregated Stack Monomer Emission. Angew. Chem. Int. Ed. 2016, 55, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Higuchi, M. A vapoluminescent Eu-based metallo-supramolecular polymer. Chem. Commun. 2012, 48, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Parker, D. A single component ratiometric pH probe with long wavelength excitation of europium emission. Chem. Commun. 2007, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, J.; Ohno, T.; Tsumatori, H.; Shiba, R.; Kamikubo, H.; Kataoka, M.; Hasegawa, Y.; Kawai, T. Fingerprint signatures of lanthanide circularly polarized luminescence from proteins covalently labeled with a β-diketonate europium(III) chelate. Chem. Commun. 2013, 49, 4604–4606. [Google Scholar] [CrossRef] [PubMed]
- SeethaLekshmi, S.; Ramya, A.R.; Reddy, M.L.P.; Varughese, S. Lanthanide complex-derived white-light emitting solids: A survey on design strategies. J. Photochem. Photobiol. C 2017, 33, 109–131. [Google Scholar] [CrossRef]
- Hai, J.; Li, T.; Su, J.; Liu, W.; Ju, Y.; Wang, B.; Hou, Y. Reversible Response of Luminescent Terbium(III)-Nanocellulose Hydrogels to Anions for Latent Fingerprint Detection and Encryption. Angew. Chem. Int. Ed. 2018, 57, 6786–6790. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Surender, E.M.; Bradberry, S.J.; Bright, S.A.; McCoy, C.P.; Williams, D.C.; Gunnlaugsson, T. Luminescent Lanthanide Cyclen-Based Enzymatic Assay Capable of Diagnosing the Onset of Catheter-Associated Urinary Tract Infections Both in Solution and within Polymeric Hydrogels. J. Am. Chem. Soc. 2017, 139, 381–388. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, L.; Vliet, K.V.; Miserez, A.; Holten-Andersen, N. White Light-Emitting Multistimuli-Responsive Hydrogels with Lanthanides and Carbon Dots. ACS Appl. Mater. Interfaces 2018, 10, 10409–10418. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, Y.; Liu, D.; Li, Z.; Li, H. Luminescence modulation via cation-π interaction in a lanthanide assembly: Implications for potassium detection. J. Mater. Chem. C 2018, 6, 1944–1950. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Snee P., T.; Somers, R.C.; Nair, G.; Zimmer, J.P.; Bawendi M., G.; Nocera, D.G. A Ratiometric CdSe/ZnS Nanocrystal pH Sensor. J. Am. Chem. Soc. 2015, 128, 13320–13321. [Google Scholar] [CrossRef] [PubMed]
- Truman, L.K.; Comby, S.; Gunnlaugsson, T. pH-Responsive Luminescent Lanthanide-Functionalized Gold Nanoparticles with “On-Off” Ytterbium Switchable Near-Infrared Emission. Angew. Chem. Int. Ed. 2012, 51, 9624–9627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-B.; Zhang, H.-Y.; Liu, Y. Dual-Stimulus Luminescent Lanthanide Molecular Switch Based on an Unsymmetrical Diarylperfluorocyclopentene. J. Am. Chem. Soc. 2013, 135, 10190–10193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, G.; Wang, Y.; Li, H. Reversible Phase Transition of Robust Luminescent Hybrid Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 2194–2198. [Google Scholar] [CrossRef]
- Biju, S.; Reddy, M.L.P.; Cowley, A.H.; Vasudevan, K.V. 3-Phenyl-4-acyl-5-isoxazolonate complex of Tb3+ doped into poly-β-hydroxybutyrate matrix as a promising light-conversion molecular device. J. Mater. Chem. 2009, 19, 5179–5187. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, D.; Zhang, L.; Jiang, K.; Qian, G. Cryogenic Luminescent Tb/Eu-MOF Thermometer Based on a Fluorine-Modified Tetracarboxylate Ligand. Inorg. Chem. 2018, 57, 12596–12602. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Fiaczyk, K.; Ramalho, J.F.C.B.; Sojka, M.; Carlos, L.D.; Zych, E. Widening the Temperature Range of Luminescent Thermometers through the Intra- and Interconfigurational Transitions of Pr3+. Adv. Opt. Mater. 2018. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Li, Z.; Li, H. Reversible On-Off Luminescence Switching in Self-Healable Hydrogels. Langmuir 2015, 31, 12736–12741. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Wang, Y.; Wang, Y.; Li, H. Luminescent europium(III)-β-diketonate complexes hosted in nanozeolite L as turn-on sensors for detecting basic molecules. Chem. Commun. 2014, 50, 13680–13682. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, P.; Xu, Q.; Li, H. Europium(III)–β-diketonate complex-containing nanohybrid luminescent pH detector. Chem. Commun. 2015, 51, 10644–10647. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Wang, Y.; Li, H. A simple and effective luminescence sensor distinguishing cationic surfactants from other type of surfactants. Sens. Actuators B 2016, 235, 206–212. [Google Scholar] [CrossRef]
- Li, P.; Li, H. Amine vapor responsive lanthanide complex entrapment: Control of the ligand-to-metal and metal-to-metal energy transfer. J. Mater. Chem. C 2016, 4, 2165–2169. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Yao, D.; Li, H. Colorimetric sensor arrays for amines based on responsive lanthanide complex entrapment. J. Mater. Chem. C 2017, 5, 6805–6811. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications-a review. Sens. Actuators B 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Gao, M.; Li, S.; Lin, Y.; Geng, Y.; Ling, X.; Wang, L.; Qin, A.; Tang, B.Z. Fluorescent Light-Up Detection of Amine Vapors Based on Aggregation-Induced Emission. ACS Sens. 2016, 1, 179–184. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Huszár, H.; Pogány, A.; Bozóki, Z.; Mohácsi, Á.; Horváth, L.; Szabó, G. Ammonia monitoring at ppb level using photoacoustic spectroscopy for environmental application. Sens. Actuators B 2008, 134, 1027–1033. [Google Scholar] [CrossRef]
- Wang, P.G.; Krynitsky, A.J. Rapid determination of para-phenylenediamine by gas chromatography-mass spectrometry with selected ion monitoring in henna-containing cosmetic products. J. Chromatogr. B 2011, 879, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Nanda, K.K. Au Nanocomposite Based Chemiresistive Ammonia Sensor for Health Monitoring. ACS Sens. 2015, 1, 55–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, C.; Ma, X.; Che, Y.; Zhao, J. Fluorescent and photoconductive nanoribbons as a dual-mode sensor for selective discrimination of alkyl amines versus aromatic amines. Chem. Commun. 2015, 51, 15004–15007. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.T.; Edwards, S.; Diamond, D. Solid-state ammonia sensor based on Berthelot’s reaction. Sens. Actuators B 2004, 98, 12–17. [Google Scholar] [CrossRef]
- Panigrahy, B.; Aslam, M.; Bahadur, D. Effect of Fe doping concentration on optical and magnetic properties of ZnO nanorods. Nanotechnology 2012. [Google Scholar] [CrossRef] [PubMed]
- Sulikowski, B.; Haber, J.; Kubacka, A.; Pamin, K.; Olejniczak, Z.; Ptaszynski, J. Novel “ship-in-the-bottle” type catalyst: Evidence for encapsulation of 12-tungstophosphoric acid in the supercage of synthetic faujasite. Catal. Lett. 1996, 39, 27–31. [Google Scholar] [CrossRef]
- Li, P.; Yang, D.; Li, H. Luminescence ethylenediamine sensor based on terbium complexes entrapment. Dyes Pigments 2016, 132, 306–309. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Kirillov, A.M.; Xie, Y.; Shan, C.; Wang, B.; Shi, C.; Tang, Y. A paper-based lanthanide smart device for acid-base vapour detection, anti-counterfeiting and logic operations. Inorg. Chem. Front. 2016, 3, 1014–1020. [Google Scholar] [CrossRef]
- Xu, J.; Jia, L.; Jin, N.; Ma, Y.; Liu, X.; Wu, W.; Liu, W.; Tang, Y.; Zhou, F. Fixed-component Lanthanide-Hybrid-Fabricated Full-Color Photoluminescent Films as Vapoluminescent Sensors. Chem. Eur. J. 2013, 19, 4556–4562. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Li, H.; Calzaferri, G. Luminescence Enhancement after Adding Stoppers to Europium(III) Nanozeolite L. Angew. Chem. Int. Ed. 2014, 53, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Li, P.; Wang, Y.; Wang, T.; Li, H. Ammonia-Responsive Luminescence of Ln3+-β-diketonate Complex Encapsulated within Zeolite Y. Molecules 2019, 24, 685. https://doi.org/10.3390/molecules24040685
Deng Y, Li P, Wang Y, Wang T, Li H. Ammonia-Responsive Luminescence of Ln3+-β-diketonate Complex Encapsulated within Zeolite Y. Molecules. 2019; 24(4):685. https://doi.org/10.3390/molecules24040685
Chicago/Turabian StyleDeng, Yuchen, Peng Li, Yige Wang, Tianren Wang, and Huanrong Li. 2019. "Ammonia-Responsive Luminescence of Ln3+-β-diketonate Complex Encapsulated within Zeolite Y" Molecules 24, no. 4: 685. https://doi.org/10.3390/molecules24040685